Artículo Científico Biotecnología Vegetal Vol. 9, No. 1: 19 - 26, enero - marzo, 2009
ISSN 1609-1841 (Versión impresa)
ISSN 2074-8647 (Versión electrónica)
Plant regeneration through somatic embryogenesis in three ethiopian Coffea arabica Lin. hybrids
Ramos RA2, Wondyifraw T1, Martínez F 2, Gonzalez ME4, Endale G3, Alemayehu T 1, Zerihun A1 *Autor para correspondencia.
1 Jimma Agricultural Research Centre (JARC), Ethiopian Institute of Agricultural Research (EIAR)
2 Estación Central de Investigaciones de Café y Cacao de Cuba
3 Ethiopian Agricultural Research Organization (EARO)
4 Instituto Nacional de Ciencias Agrícolas (INCA). e-mail: esther@inca.edu.cu
RESUMEN
Genetistas del Jimma Agricultural Research Center (JARC), Etiopía, desarrollaron tres híbridos de Coffea arabica Lin.: Aba Buna, Ghawe y MCH2. Sin embargo, la multiplicación es el cuello de botella que ha impedido su distribución a los productores. Este trabajo se realizó en el laboratorio de biotecnología del JARC, con el objetivo de regenerar estos híbridos a través de embriogénesis somática. Combinaciones de ANA, AIB, Kinetina y Acido Giberélico fueron evaluadas en la formación y proliferación de callos; inducción, desarrollo y crecimiento de embriones somáticos. Las plantas fueron transferidas a condiciones de aclimatización en una mezcla de suelo y pulpa de café (1:1). Las mejores respuestas en la formación de callos, se observaron en los explantes a partir de hojas colectadas en el primer y segundo nudo en las plantas de café; siendo el medio de cultivo MS con 2,4-D (3 mg.l-1) y BA (3 mg.l-1) el de mayor porcentaje (95%), de los cuales el 45% fueron embriogénicos. Los callos con mayor masa fresca fueron observados en Aba Buna (2.80 g/explante, Ghawe (1.22 g/explante y MCH2 (0.47 g/explante respectivamente. La mayor cantidad de embriones en etapa de corazon por explante se obtuvieron con la combinación de ANA (0.1 mg.l-1) y Kinetina (0.1 mg.l-1). En el medio de cultivo con ANA (0.1 mg.l-1) y Kinetina (0.5 mg.l-1) se obtuvo el mayor número de embriones totales. El uso de medio de cultivo líquido favoreció el desarrollo de los embriones somáticos y redujo los costos en la producción in vitro de las plantas. Las plantas en aclimatización mantuvieron buena sanidad y crecimiento.
Palabras clave: callogénesis, conversión a planta, embriones somáticos, regeneración de embriones, reguladores del crecimiento
ABSTRACT
Breeders at Jimma Agricultural Research Center (JARC), Ethiopia, developed three arabica coffee hybrids: Aba Buna, Ghawe and MCH2 that exhibited heterosis over their parents. However, their distribution to farmers had been a bottleneck. This work was carried out at the plant biotechnology laboratory of JARC, with the objective of regenerating these hybrids through somatic embryogenesis. Different combinations of NAA, IBA, Kinetin and Gibberelic acid were tested for callus formation and proliferation, somatic embryo induction, as well as embryo development and growth. In vitro rooted plantlets having 2 to 5 leaf pairs were transferred to acclimatization conditions in seedling trays filled with sterile soil and well decomposed coffee pulp (1:1). Best responding leaf explants for callus formation were those collected from the first and second nodes (top to bottom) of coffee seedlings; MS medium supplemented with 2,4-D (3 mg.l-1) and BA (3 mg.l-1) the best, with 95%, of which 45% were embryogenic. The highest callus fresh weight was recorded from Aba Buna (2.80 g/explant), followed by Ghawe (1.22 g/ explant) and MCH2 (0.47 g/explant). The highest number of heart-shaped somatic embryos per explants was obtained with NAA 0.1 mg.l-1 and Kinetin 0.1 mg.l-1. Culture medium containing a combination of NAA (0.1 mg.l-1) and Kinetin (0.5 mg.l-1) favored the highest number of total somatic embryos. The use of liquid media was beneficial for somatic embryo development. It also reduced the cost of plantlet production. Plants under acclimatization retained proper growth and sanitary conditions.
Key words: callogenesis, embryo regeneration, plant conversion, plant growth regulators, somatic embryos
Abbreviations: BA (6-benzyladenine); 2,4-D (2,4-dichlorophenoxyacetic acid); IBA (indole-3-butyric acid); MS (Murashige and Skoog); NAA (naphthaleneacetic acid), GA3 (Gibberellic acid), CV (Coefficient of variation), S.E (Standard error).
INTRODUCTION
Coffee (Coffea arabica Lin.) is indigenous to Ethiopia. Forests in the Southwest of the country are the primary center of origin and center of genetic diversity of Coffea arabica Lin. (Werede, 1984; Feyera 2006). Regardless of the current fall of the world coffee price, the crop still stands as the most important export crop of the nation. This clearly suggests the need for improvement of its production, productivity and quality in order to stabilize the income from its export.
This is well recognized by breeders at Jimma Agricultural Research Center (the center of excellence for coffee research in Ethiopia), who developed 3 Coffea arabica Lin. hybrids: Aba Buna, Ghawe and MCH2. They exhibited positive heterosis over both their parents in most of the economic and agronomic characters. However, their multiplication had remained a serious bottleneck that hampered the wide dissemination to farmers.
Diverse methods have been used to solve the problem regarding multiplication of Coffea arabic Lin. hybrid such as: hand pollination, vegetative propagation (by grafting and rooting of cuttings) and somatic embryogenesis.
Although, Coffea arabica Lin. is a predominantly self-pollinated crop, propagation by seed is the common method and homogeneity within the pure-line population is adequate. However, due to segregation in the F2 generation, true-to-type hybrid propagation is not recommended through seeds. Although, the hand pollination and vegetative propagation using grafting and cutting methods provide successful cloning of arabica coffee trees; they are labour intensive, time consuming and have their own limitations for mass production of seedlings from these elite clones to meet the current wide demand. Moreover, clonal propagation through somatic embryogenesis permits the production of uniform plants on a massive scale in a relatively shorter period than using the former methods.
Etienne et al. (2002a; 2002b) stated that somatic embryogenesis had been proved much more efficient for micropropagating different species and genotypes of coffee than traditional multiplication methods. Tissue culture is the most practical and efficient propagation technique for elite arabica coffee hybrids and pure lines, as it provides the most viable alternative for rapid and large scale multiplication and dissemination of the desired material in the shortest time possible. It enables all year round seedling provision and can also be used as a tool to obtain disease-free planting materials (Ahloowalia et al., 2004; Briones and Sotomayor, 2006). Somatic embryogenesis is currently used widely for large scale propagation of Coffea species in different countries of Africa, Asia and Latin America (Etienne, 2006).
The objective of the present research was therefore to evaluate the regeneration of the three potential ethiopian arabica coffee hybrids (Aba Buna, Ghawe and MCH2) through somatic embryogenesis.
MATERIAL AND METHODS
The research was conducted at the Plant Biotechnology lab of Jimma Agricultural Research Centre (JARC), Ethiopia, between September 2005 and Jun 2007. Seedlings of the three hybrids were raised at the plant biotechnology nursery from hand pollinated seeds obtained from the Coffee Breeding division of JARC. It was later confirmed by the breeders for their true-to-typeness.
Young, fully unfolded and healthy leaves from actively growing and vigorous coffee seedlings were collected from four different nodal positions (i.e. first, second, third and fourth nodes from top to bottom) and used as explant source in culture initiation. They were thoroughly washed in the laboratory with commercial liquid soap and tap water, so as to clean the fissures all over the leaf surface; rinsed for 5 minutes with tap water; and subsequently for 3 minutes using distilled water. Later, the leaf explants were sterilized aseptically under the laminar flow hood cabinet using three different rates of two disinfectant solutions: local bleach, Berekina, (at 15, 20 and 25% for 25 minutes) and Sodium hypochlorite (at 10, 15 and 20% for 25 minutes). Then, explants were rinsed from three to four times using sterilized distilled water with cysteine (25mg.l-1). Disinfected leaves were cut into small pieces of 1 cm2 prior to incubation.
Leaf pieces were cultured on a plant growth regulators (PGR)-free semi-solid (7g.l-1 agar) incubation medium composed of ½ MS basal salts and vitamins and sucrose (30g.l-1). The pH was adjusted to 5.75 before autoclaving. A total of eight leaf pieces were incubated in each Petri dish filled with 17ml of the incubation medium, and kept in dark at 27 ± 2ºC for 72 hours.
Callus formation
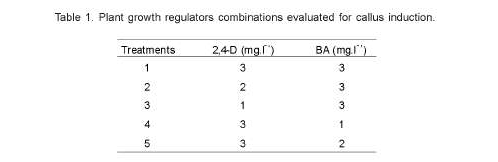
Different combinations of plant growth regulators were tested for callus induction (Table 1) under dark conditions at 27 ± 2ºC. Callus formation and embryogenic calli percentages, together with fresh weights of the produced calli, were evaluated after 10 weeks of culture. In all cases, stereoscopic microscope was used to evaluate the percentages of embryogenic calli formation.
Somatic embryos induction
Eight combinations of NAA, IBA and Kinetin were evaluated for somatic embryo induction (Table 2). Cultures were kept under cool-white fluorescent lamps providing approximately 30 μmole m2 s1 photon flux density over a 9h photoperiod and temperature of 32 ± 2ºC. The number of embryos at each stages of growth (globular, heart, torpedo and cotyledonal) were recorded from each treatment after 16 weeks of culture on the different embryo induction media tested.
Somatic embryo development and growth
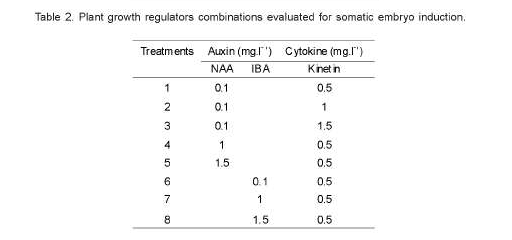
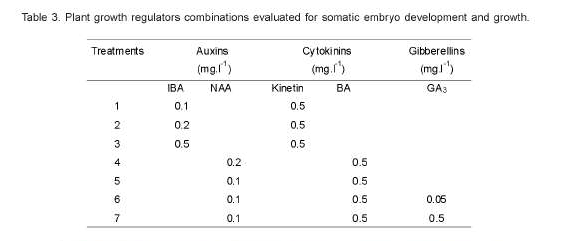
Once somatic embryos were formed they were separated from the callus and transferred onto a medium for subsequent development and growth (Table 3). The environmental conditions adopted were temperatures of 32 ± 2ºC and 9 hours light (40 μmole m2 s1 photosynthetic photon flux) from cool florescent lamps. The parameters evaluated were stem length (cm) and number of leaves. The semi-solid medium that showed best results, for development and growth of somatic embryos, was further tested using liquid culture on a shaker at 100 rpm.
Acclimatization
In vitro rooted plantlets with 2 to 5 pairs of leaves were subjected to acclimatization on seedling trays filled with sterile soil and well decomposed coffee pulp at a 1:1 ratio. They were kept under a transparent poly sheet tunnel in greenhouse for the first 10 days, before transferring to nursery conditions. The experiment was undertaken in a completely randomized Design (CRD) with fifty replicates per treatment and five explants per flask under each replicate. Statistical analyses were carried out using ANOVA and the Duncan multiple range tests was used for mean separation. In all cases, the analyses were performed using the Statistica v5.1
RESULTS AND DISCUSSION
Complete disinfection (100%) of leaf explants was attained using 20% (v/v) sodium hypochlorite solution
for 25 minutes, or a 25 minutes treatment with 25% local bleach (Berekina) solution. The use of Berekina at the stated rate and duration seems feasible for future commercial use. It is a cheaper and commonly available local product at any supermarket in the country.
Callus formation
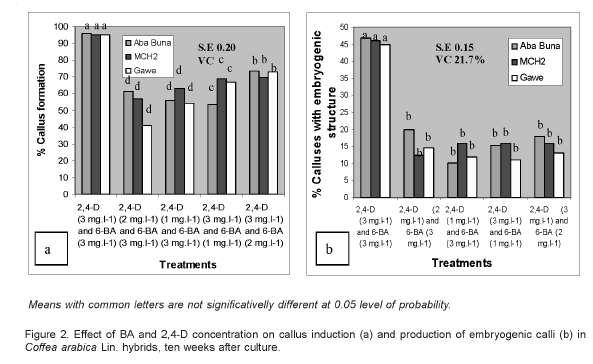
After 3 to 4 weeks of transferring to the appropriate medium, creamy colored calli were induced along the cut surfaces of the leaf piece explants (Figure 1a and 1b). The best results in callus induction were obtained from leaves found on the first and second nodes (top to bottom) on the coffee seedlings under nursery conditions. Leaves from these positions are generally healthy and physiologically active. Subsequently, these calli underwent further proliferation after 6 to 10 weeks of culture thereby covering most of the explant surface and their color also changed to brown (Figure 1a and 1b).
Highly significant differences were observed on total percentages of callus induction and the percentage of calli with embryogenic potential for the different culture media compositions tested.
Among the different media types tested, the combination of 2,4-D and BA each at 3 mg.l-1 (Treatment 1) was the best, achieveing a 95% callus induction, of which 45% were embryogenic. No significant differences were observed between the three coffee hybrids tested for either callus induction or embryogenic calli induction frequencies.
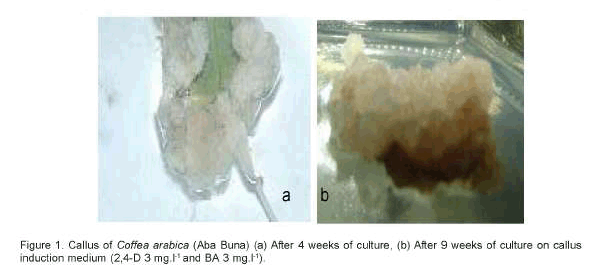
However, the three coffee hybrids tested in this experiment had revealed significant differences in callus fresh weight when cultured on the best callus induction medium, i.e. treatment 1 (2,4-D 3mg.l-1 and BA 3mg.l-1). The highest callus fresh weight was recorded from Aba Buna (2.8g/explant), followed by Ghawe (1.22g/explant), while the least was from MCH2 (0.47g/explant).
The results indicate around 45% embryogenic responses from cultured leaf explants, in contrast to Figueroa et al. (2002) who was able to get around 70%. All these differences could be attributed to the genotype of the materials used in the two cases. This isdirectly agree with the statements of several researchers (Santana et al., 2004; Gatica et al., 2007).
Sondahl and Sharp (1977) had also reported strong interactions between different plant growth regulators on callus induction, proliferation and subsequent regenerations in Coffea arabica. As reviewed by Etienne et al. (2004), several reports in different parts of the world had also confirmed the efficacy of BA in combination with 2,4-D in callus induction and proliferation of arabica coffee, in agreement with the current result.
Somatic embryo induction
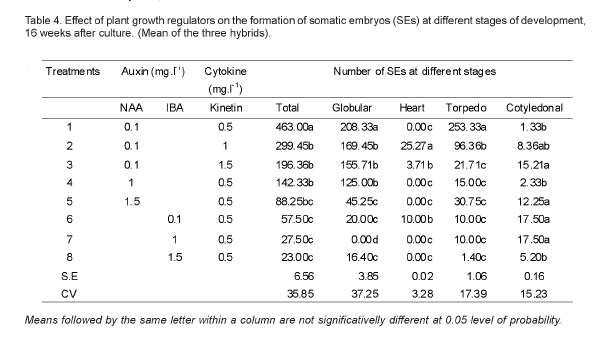
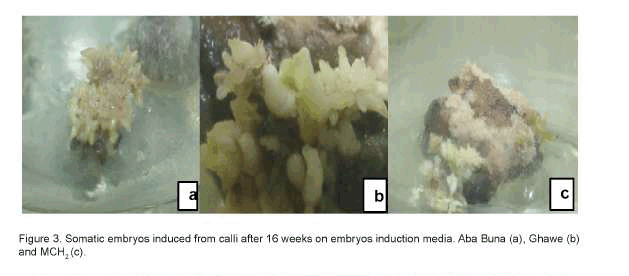
Statistically significant differences (P<0.01) were observed among treatments, but not among the three hybrids, for the number of somatic embryos produced per explant. Culture treatment-1, containing a combination of NAA (0.1mg.l-1) and Kinetin (0.5mg.l-1) developed the highest number of somatic embryos of the three hybrids and all the treatment combinations evaluated. Likewise, significant differences were observed between treatments regarding the number of somatic embryos at the heart stage of development (Table 4). In the three ethiopian arabica coffee hybrids, somatic embryos were successfully induced after 16 weeks of culture the embryogenic calli on embryo induction media (Figure 3). Therefore, the highest number of heart-shaped somatic embryos per explants were obtained from treatment 2 (NAA 0.1mg.l-1 and Kinetin 0.1mg.l-1), followed by treatments 3 (NAA 0.1mg.l-1 and Kinetin 1.5mg.l-1) and treatment 6 (IBA 0.1mg.l-1 and Kinetin 0.5mg.l-1), while none of the rest five treatments resulted in heart-shaped somatic embryos after 16 weeks of incubation. The number of torpedo-shaped somatic embryos also showed statistically significant differences (p<0.01) with the type of culture medium used. Therefore, treatment 1 (NAA 0.1mg.l-1 and Kinetin 0.5mg.l-1) offered, comparatively, the largest number of torpedo-shaped somatic embryos (253.33/ explant), followed by treatment 2 (96.36/explant). On the other hand, the highest number of cotyledonal stage somatic embryos were recorded from treatments 6 (IBA 0.1mg.l-1 and Kinetin 0.5mg.l-1) and 7 (IBA 1mg.l-1 and Kinetin 0.5mg.l-1) (Table 4).
The current result was in agreement with Sondahl and Sharp (1977) who stressed the benefits of using NAA and Kinetin combined for successful embryogenesis in coffee.
As the somatic embryos were formed on the surfaces of the calli, it was essential to fragment them into smaller peaces to enhance their contact with the culture medium and thereby enhance production of somatic embryos. Based on the differences in their stages of development and morphology, the somatic embryos produced in the process were of different types, i.e. globular, heart, torpedo and cotyledonal. This was in direct agreement with the reports of Flota and Vargas (2003), who observed six different developmental stages of somatic embryos in Coffea arabica in the course of somatic embryogenesis.
Somatic embryos development and growth
Somatic embryos were separated from the callus once they were well formed. Then, they were transferred either to solid (Figure 4) or liquid media (Figure 5) for further growth and development. On the average, this phase of somatic embryo growth and development took around 30 to 45 days. The difficulty in separating individual somatic embryos from the callus mass and the relatively high frequency of secondary embryogenesis were some of the side effects observed in using the solid medium, which restricts its use for mass propagation at commercial level.
On the other hand, growth and development of somatic embryos in liquid medium, using orbital shaker (100 rpm), showed to be better than in solid medium (Figure 5). In general, the peculiar benefit from the use of liquid medium emanates from the improved contact between the culture medium and the explants. This situation facilitates uptake of nutrients and hence results in enhanced growth and development of somatic embryos. The use of liquid media is also relatively cheaper, as it avoids the cost incurred for solidifying agents. It is also praised for facilitating the separation of somatic embryos from their maternal tissue, which allows better establishment at subsequent culture stages. This result was in agreement with the statements of Debergh (1988) and Aitken-Christie (1991) who stated the positive impacts of liquid media in the development of coffee plantlets from somatic embryos. They had also reported liquid cultures to be ideal in micropropagation, as they greatly reduce costs of plantlet production and enable automation.
No statistical differences were observed in growth and development of plantlets from most of the treatments evaluated in this experiment, except for treatment 5 (NAA 0.1mg.l-1 and BA 0.5 mg.l-1) (Table 5). Therefore, compared to the rest five treatments, the shortest shoot and least number of leaves were recorded from treatment 5 (NAA 0.1mg.l-1 and BA 0.5mg.l-1), though the results from this treatment combination had no statistical difference with those of treatment 3 (IBA 0.5 mg.l-1 and Kinetin 0.5mg.l-1) and treatment 1 (IBA 0.1mg.l-1 and Kinetin0.5 mg.l-1). However, the highest results for both parameters were recorded from treatment 6 (NAA 0.1 mg.l-1, BA 0.5 mg.l-1 and AG3 0.05mg.l-1).

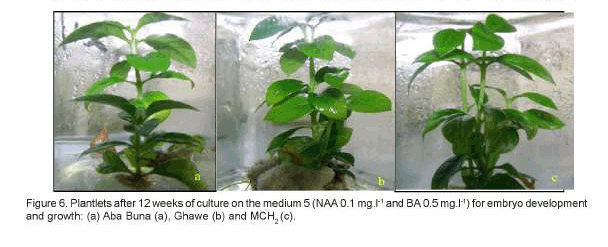
Statistically significant differences (P<0.01) were observed among the hybrids for the number of leaves and shoot length from the use of different media compositions. Therefore, of the three hybrids evaluated, plantlets from Ghawe produced the highest number of leaves (7.90) and longest shoots (2.51cm), followed by MCH2 (7.58 leaves and 2.25cm shoot). While plantlets from Aba Buna showed the least records for both parameters (6.34 leaves and 1.78cm shoot). The hybrid MCH2, out of the tree, had revealed a tendency of multiple shoot production under treatment 5 (NAA 0.1mg.l-1 and BA 0.5mg.l-1), as can be observed in Figure 6, unlike the other two (Aba Buna and Ghawe). The effect of BA inducing 4 sprouting per nodal explants of C. arabica cv Catimor (Jesus et al., 2003) was verified in similar studies.
Acclimatization
Plantlets with 2 to 5 pairs of leaves were later transferred to ex-vitro condition for acclimatization retaining proper phytosanitary conditions. Acclimatizing plantlets had good growth on a sterilized 1:1 potting mix of forest soil and well decomposed coffee pulp (Figure 7).


CONCLUSIONS
Plant regeneration through indirect somatic embryogenesis in the three ethiopian Coffea arabica Lin hybrids (Aba Buna, Ghawe and MCH2) had been achieved. The liquid media for the growth and development of somatic embryos is relatively cheaper and decrease the possibility of secondary embryogenesis.
ACKNOWLEDGMENTS
The authors wish to acknowledge all the supports rendered by W/ro Roman Getachew and all other members in the Biotech lab for the successful completion of the study. We also extend our sincere appreciation and thanks to Mr. Million Abebe, the former Center Director of JARC, who had supported and encouraged us throughout the experimental period.
REFERENCES
Ahloowalia, BS, Prakash J, Savangikar VA, Savangikar C (2004) Plant Tissue Culture. In: Proceedings of a Technical Meeting Organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, pp 3-10. FAO Vienna
Aitken-Christie, J (1991) Automation. In: Debergh PC and Zimmerman RJ (Eds) Micropropagation: Technology and Application, pp. 363-388. Kluwer Academic Publishers, Dordrecht
Briones, SCDL, Sotomayor HSMT (2006) Coffee Biotechnology Braz. J. Plant Physiol 18 (1):217-227
Debergh, P (1988) Improving mass propagation of in vitro plantlets. In: Kozai T (Ed) Horticulture in High Technology Era, pp. 45-57. International Symposium on High Technology in Protected Cultivation, Tokyo
Etienne, DB, Bertrand B, Schlönvoigt A, Etienne H (2002a) The morphological variability within a population of coffee somatic embryos produced in a bioreactor affects the regeneration and the development of plants in the nursery. Plant Cell Tissue Organ Cult 68:153-162
Etienne, DB, Bertrand BN, Vasquez, Etienne H (2002b) Comparison of somatic embryogenesis-derived coffee (Coffea arabica L.) plantlets regenerated in vitro or ex-vitro:
Morphological, mineral and water characteristics. Ann of Bot. 90: 77-85
Etienne, H (2006) Somatic embryogenesis protocol: Coffee (Coffea arabica L. and C. canephora P.). In: Jain SM, Guta PK (eds). Protocol for somatic embryogenesis in woody plants, pp.167-179, Springer.
Etienne, H, Alpizar E, Dechamp E, Bertrand B (2004) Agronomic Performance and Trueness-to-type of Coffea arabica Hybrids Mass-propagated by Somatic Embryogenesis. In: Proceedings of the 20th ASIC conference, pp. 897 907.Bangalore, India
Feyera, S (2006) Biodiversity and ecology of afromontane rainforests with wild Coffea arabica L. populations in Ethiopia Ph.D Thesis, pp. 144. University of Bonn/Germany
Figueroa, FRQ, Cerda CFJF, Herrera RR, Vargas VML (2002) Histological studies on the developmental stages and differentiationof two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep 20:11411149
Flota, VA, Vargas VM (2003) In vitro plant cell culture as the basis for the development of a Research Institute in Mexico: Centro de Investigación Científica de Yucatan. In Vitro Cell. Dev. Biol. Plant 39: 250258
Gatica, AM, Arrieta G, Espinoza AM (2007) Comparison of three in vitro protocols for direct somatic embryogenesis of and plant regeneration of Coffea arabica L. CVS. Caturra and Catuai. Agronomía Costarricense 31(1): 85-94
Jesus, AMS, Carvalho SP, Pasqual M, Carvalho M, Correa LVT (2003) Efeitos de diferentes concentrações de BAP e dos meios básicos MS e WPM na proliferação e desenvolvimento de brotos axilares Coffea arabica in vitro. In: SIMPÓSIO DE PESQUISAS DOS CAFÉS DO BRASIL, 3, 2003, Porto Seguro. Anais. Brasília:Embrapa-Café, p.93
Santana, N, González ME, Valcárcel M, Canto-Flick A, Hernández M, Fuentescerda CFJ, Barahona F, Cortés J, Loyola-Vargas VM (2004) Somatic embryogenesis: a valuable alternative for propagating selected robusta coffee (Coffea canephora) clones. In Vitro Cell. Dev. Biol.-Plant 40:95-101
Sondahl, MR, Sharp WR (1977) Interaction of cytokinin and auxin in growth and embryogenesis of cultured leaf of Coffee. In: International conference on regeneration of developmental process in plants. Halle, pp.18
Werede, M (1984) Coffee genetic resources in Ethiopia conservation and utilization particular reference to CBD resistance. In: Proc. 1* Reg. Worksh. CBD, p. 203-211. Addis Ababa/Ethiopia
Copyright (c) 2016 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
