COMUNICACIÓN CORTA
Biotecnología
Vegetal Vol. 15, No. 3: 187 - 191 julio - septiembre, 2015
ISSN 2074-8647, RNPS: 2154 (Versión electrónica)
Instituto de Biotecnología de las Plantas. UCLV. MES.
In vitro propagation in Temporary Immersion System of sugarcane plants variety `RB 872552' derived from somatic embryos
Propagación in vitro en Sistemas de Inmersión Temporal de plantas de caña de azúcar variedad `RB 872552' obtenidas de embriones somáticos
Marina Medeiros de Araújo Silva*, Emmanuel Cabral Medeiros, Gileno Vitor Mota Lima, Lilia Willadino, Terezinha Camara. *Author for correspondence
Universidade Federal Rural de Pernambuco. Rua Dom Manoel de Medeiros, S/N, Dois Irmãos. Recife. Pernambuco. Brasil. CEP 52171-900. e-mail: marinamedeirosas@yahoo.com.br
ABSTRACT
In this study, we used a temporary immersion system (TIS) to multiply sugarcane (Saccharum spp.) plants obtained by somatic embryogenesis (SE). SE was induced from immature leaf segments that were grown in culture medium supplemented with 2,4-D and BAP. Embryo formation occurred in 81% of the inoculated explants and 254 plants were regenerated. Ninety plants were transferred to TIS and cultured in medium supplemented with BAP. After three subcultures, 60 000 plantlets were obtained and transferred to rooting media. After 30 days of acclimatization period plantlets were well developed and exhibited a 96% survival. The results demonstrate the feasibility of the combined use of two important techniques of in vitro culture (SE and shoot multiplication in TIS) to sugarcane in vitro propagation.
Key words: acclimatization, 6-benzylaminopurine, Saccharum spp.
RESUMEN
En este estudio se utilizaron sistemas de inmersión temporal (SIT) para la multiplicación de plantas de caña de azúcar (Saccharum spp.) obtenidas por embriogénesis somática (ES). La ES fue inducida en segmentos de hojas inmaduras cultivadas en medio de cultivo con 2,4-D y BAP. La formación de embriones ocurrió en el 81% de los explantes inoculados y se regeneraron 254 plantas. A los SIT se transfirieron 90 plantas que fueron cultivadas en medio de cultivo con BAP. Después de tres subcultivos, se obtuvieron 60 000 plantas que fueron transferidas a medio de cultivo de enraizamiento. Las plantas mostraron un buen desarrollo, con una supervivencia del 96% después de 30 días de aclimatización. Los resultados demostraron la viabilidad del uso combinado de dos técnicas de cultivo in vitro (ES y la multiplicación de brotes en SIT) en la propagación in vitro de caña de azúcar.
Palabras clave: aclimatización, 6-bencilaminopurina, Saccharum spp.
INTRODUCTION
Somatic embryogenesis (SE) is the primary method used for Saccharum spp. improvement through genetic engineering (Lakshmanan et al., 2005). SE has proven to be more efficient for the generation of transgenic plants than organogenesis (Desai et al., 2004; Singh et al., 2013). However, for commercial micropropagation of sugarcane, plants are typically regenerated through organogenesis from shoot tips in conventional culture (static) and in temporary immersion system (TIS) (Lemos, 2009).
The advantages of TIS for growth and development of plants have been demonstrated in different species of commercial importance, such as Musa spp. (Roels et al., 2005), Phalaenopsis and Cymbidium orchids (Tirado et al., 2005; Gao et al., 2014) and Ananas comosus (Silva et al., 2007), besides, the shoot multiplication of sugarcane (Lorenzo et al., 1998; Mordoco et al., 2009).
In micropropagation protocols, the multiplication phase, which includes numerous subculturing steps, consumes the greatest amount of time and resources in the laboratory. The acclimatization stage should be performed carefully, taking into account the high commercial value of plantlets. Furthermore, micropropagated plantlets should be handled in order to ensure their survival and full development under environmental conditions of nurseries and greenhouses, which differ extensively from the in vitro conditions (Chandra et al., 2010).
This study describes the multiplication in TIS and the acclimatization of sugarcane plants, variety `RB 872552', generated by SE.
MATERIALS AND METHODS
Plant material
Plants of sugarcane (variety `RB 872552') previously established in vitro were selected and transferred to MS (Murashige and Skoog, 1962) culture medium without growth regulators. After 40 days, the plants were used to induce SE.
Somatic embryogenesis
Somatic embryos were obtained from immature leaf fragments grown in MS culture medium supplemented with 3% (w/v) sucrose, 13.6 µM 2,4-Dichlorophenoxyacetic acid (2,4-D) and 1.1 µM 6-benzylaminopurine (BAP), 6 g l-1 agar, and pH 5.8. The explants were kept in a growth room at 25 ± 2 °C with a photoperiod of 16 h and a luminous intensity of 47 µmol m2 s-1, during 40 days (Silva et al., 2014).
After the conversion phase, which was accomplished through the suppression of growth regulators in the culture medium, the number of regenerated plants was quantified.
Multiplication in TIS
Ninety regenerated plants (> 2 cm, measured with a ruler from the base of stem until the last expanded leaf) were transferred to three TIS (30 plants per TIS), each with a 5 liters capacity, and containing MS medium (pH 5.8) supplemented with 3% sucrose and 0.9 µM BAP. The TIS were incubated under a 25 ± 2°C, photon flux density of 47 µmol m-2 s-1, 16 h photoperiod and immersion frequency of 5 min every 3h. After 8 weeks, the secondary shoots (tillers) generated were subcultured to a new TIS (50 tillers per TIS), which were maintained under the culture conditions described above. Two other subcultures were performed every 4 weeks, and the number of plants formed and multiplication rate were recorded.
Rooting and acclimatization
After the multiplication stage the tillers were transferred to TIS containing rooting medium composed of MS salts with 53.7 mM á-naphthaleneacetic acid (NAA) and were maintained during 15 days under same culture conditions described above. Afterward, plants with an average height of 9.5 cm, fresh mass of 0.326 g, average of four leaves per plant and presence of roots, were transferred to a greenhouse (30 °C and 56-80% relative humidity). The substrate was composed of sugarcane bagasse, sand and clay (1:1:1 ratio), and irrigation was performed three times a day (morning, afternoon and night) to the point of drainage. The plants were analyzed every 15 days for stem and root length, and basal stem diameter using a ruler and a digital caliper, respectively, and the number of leaves was counted. The fresh biomass of aerial and root parts were weighed, and then the parts were dried in a drying oven (60°C) to a constant mass. The survival rate and tillering were assessed after 30 days of acclimatization stage.
The experiment was conducted in a completely randomized design. Sixty replicates were used for ES induction, and 50 plants were evaluated every 15 days (until 30 days) during acclimatization. The data were analyzed by descriptive analysis.
RESULTS AND DISCUSSION
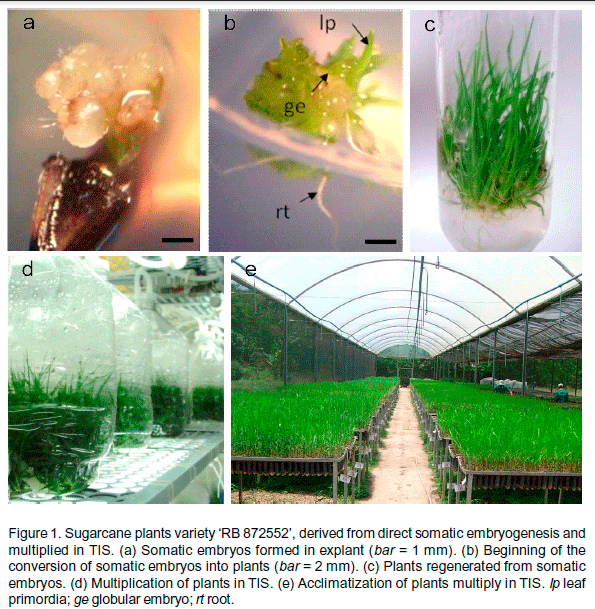
The initial formation of somatic embryos occurred on the 8th day. After 40 days, 81% of inoculated explants showed a positive response with somatic embryos developed directly from leaf tissue (Fig. 1a). At the end of conversion stage, within 90 days after inoculation, 254 plants were regenerated (Fig. 1b, c).
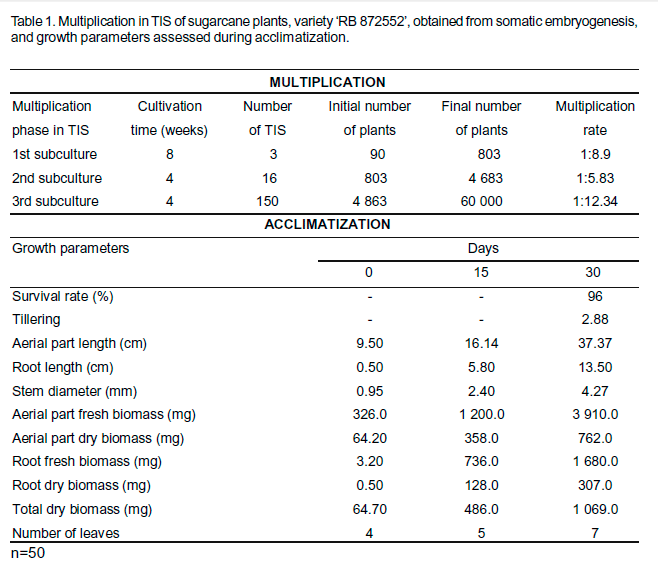
After subcultures in multiplication medium, 150 TIS (Fig. 1d) containing approximately 400 plants each, totaling 60 000 plants, were obtained (Table 1). In the last subculture, it was obtained a multiplication rate of 1:12.34, demonstrating the effectiveness of the TIS use. Yadav et al. (2014), studying the effect of trimming of propagules on the rate of shoot multiplication of sugarcane, obtained 1:6.0 as the higher average, using shoot clumps with 3.0 cm of length and conventional culture in flasks.
At the end of the multiplication and rooting phases, which lasted approximately 130 days, the plants showed adequate developed (size, number of leaves, coloration, and root system) and were acclimatized (Fig. 1e). Because of increased automation, reduced costs, enhanced production rate and a high quality of plant material, TIS offers many advantages over conventional micropropagation procedures (Lima et al., 2012). The increased of multiplication rate and the marked improvements in the quality of the plants generated in TIS are due, in part, to the regular supply of nutrients and oxygen (Watt, 2012).
After 30 days of acclimatization, the plants had a 96% survival rate and exhibited an increase in all of the growth parameters assessed (Table 1). The rate of tillering was 1:2.88. Thus, from the 5 400 plants that were planted in greenhouse, 15 552 plants suitable for planting in the field were obtained. Plantlets quality is extremely important because it influences survival and growth rates. Therefore, to obtain fully-grown, well-developed healthy plants, the control of limiting factors, such as light, temperature and humidity, are basic conditions that are necessary for successful acclimatization (Souza et al., 2006).
This study demonstrates that the induction of direct SE in sugarcane `RB 872552' combined with the use of TIS is a promising alternative for large-scale multiplication of plants regenerated from SE. The successful application of these propagation techniques was corroborated by high survival rate of plants after the acclimatization stage, implying that this method can be used with positive effect in other varieties or species of commercial interest.
ACKNOWLEDGMENTS
The authors would like to thank the Biofábrica Governador Miguel Arraes of CETENE (Centro de Tecnologias Estratégicas para o Nordeste, Recife, PE, Brazil) for providing the plants and bioreactors used in this study.
REFERENCES
Chandra S, Bandopadhyay R, Kumar V, Chandra R (2010) Acclimatization of tissue cultured plantlets: from laboratory to land. Biotechnol Lett 32: 1199-1205
Desai NS, Suprasanna P, Bapat VA (2004) Simple and reproducible protocol for direct somatic embryogenesis from cultured immature inflorescence segments of sugarcane (Sacharum spp.). Curr Sci 87: 764-768
Gao R, Wu SQ, Piao XC, Park SY, Lian ML (2014) Micropropagation of Cymbidium sinense using continuous and temporary airlift bioreactor systems. Acta Physiol Plant 36: 117-124
Lakshmanan P, Geijskes RJ, Aitken KS, Grof CLP, Bonnett GD, Smith GR (2005) Sugarcane biotechnology: the challenges and opportunities. In Vitro Cell Dev Biol Plant 41: 345-363
Lemos EEP (2009) Micropropagação de plantas por Biorreatores. In: Junghans TG, Souza AS (Eds.) Aspectos práticos da micropropagação de plantas, pp. 83-119. Embrapa Mandioca e Fruticultura Tropical. Cruz das Almas
Lima GPP, Campos
RAS, Willadino L, Câmara TJR, Vianello F (2012) Polyamines, gelling agents
in tissue culture, micropropagation of medicinal plants and bioreactors. In:
Leva A, Rinaldi LMR (Eds.) Recent advances in plant in vitro culture.
InTech. Rijeka
Lorenzo JC, González BL, Escalona M, Teisson C, Espinosa P, Borroto C (1998)
Sugarcane shoot formation in an improved temporary immersion system. Plant Cell
Tiss Organ Cult. 54: 197-200
Mordocco AM, Brumbley JA, Lakshmanan P (2009) Development of a temporary immersion system (RITA®) for mass production of sugarcane (Saccharum spp. interspecific hybrids). In Vitro Cell Dev Biol Plant 45: 450-457
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 15: 473-497
Roels S, Escalona M, Cejas I, Noceda C, Rodriguez R, Canal MJ, Sandoval J, Debergh P (2005) Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell Tiss Organ Cult. 82: 57-66
Silva AB, Pasqual M, Teixeira JB, Araújo AG (2007) Métodos de micropropagação de abacaxizeiro. Pesq Agropec Bras. 42: 1257-1260
Silva MMA, Ulisses C, Medeiros MJL, Granja MMC, Willadino L, Camara T (2014) Antioxidant enzymes activity in embryogenic and non-embryogenic tissues in sugarcane. Acta biol. Colomb. 19: 75-82
Singh RK, Tiwari NN, Rastogi J, Singh SP (2013) Current status of sugarcane transgenic: an overview. Adv Genet Eng 2: 1-7
Souza FVD, Costa MAPC, Neto HPS (2006) Aclimatização. In: Junghans TG, Souza AS (Eds) Introdução à Micropropagação de plantas, pp. 131-139. Embrapa Mandioca e Fruticultura Tropical. Cruz das Almas
Tirado JM, Naranjo EJ, Atehortúa L (2005) Propagación in vitro de Phalaenopsis (Orchidaceae) a partir de protocormos, mediante el sistema de inmersión temporal RITA. Rev Colomb Biotecnol 7: 25-31
Watt MP (2012) The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechnol. 11: 14025-14035
Yadav S, Ahmad A, Rastogi J, Lal M (2014) Effect of propagule trimming on shoot multiplication rate in sugarcane micropropagation. J. Sugarcane Research 4: 82-85
Recibido: 26-06-2015
Aceptado:
20-08-2015
Copyright (c) 2016 Biotecnología Vegetal
![]()
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.