Artículo original
Biotecnología
Vegetal Vol. 14, No. 2: 67 - 71, abril - junio, 2014
ISSN
2074-8647, RNPS: 2154 (Versión electrónica)
Instituto
de Biotecnología de las Plantas. UCLV. MES.
Scaling-up the biomass production of Cymbopogon citratus L. in temporary immersion system
Escalado de la producción de biomasa de Cymbopogon citratus L. en biorreactores de inmersión temporal
Elisa Quiala*, Raúl Barbón, Alina Capote, Naivy Pérez-Alonso, Maité Chávez, Manuel de Feria, Elio Jiménez *Author for correspondence
Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera de Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba. CP 54830. e-mail: elisa@ibp.co.cu
ABSTRACT
Shoot-tips, collected from greenhouse-grown plants of Cymbopogon citratus L. (lemmon grass), were incubated on a semi-solid Murashige and Skoog (MS) medium with 30% (w/v) sucrose, and supplemented with 0.89 µM 6-benzyladenine (BA). After three weeks of culture shoots were individualized and then inoculated in 10 litres temporary immersion system (TIS) containing 3 litres of the same basal MS liquid medium. The effects of three immersion frequency (immersion every 12, 6 and 4 hours) on the production of biomass were studied. Three inoculum densities (forty, fifty and sixty shoots/TIS) were also tested. The biomass growth was inûuenced by the immersion frequency. The highest proliferation rate (17.3 shoots/explants) and the plant length (45.2 cm) were obtained in plants immersed every 4 h. Also, the fresh and dry biomass weight (153.4 gFW and 24.8 gDW, respectively) were higher in this treatment. The maximum biomass accumulation (185.2 gFW and 35.2 gDW) was achieved after 30 days of culture when an inoculum density of 60 explants per TIS was used. For the first time, biomass of C. citratus has been produced in10 litres TIS. These results represent the first step in the scaling-up the biomass production of this medicinal plant in large temporary immersion bioreactors.
Key words: automation, biomass growth, lemmon grass medicinal plant, tissue culture
RESUMEN
Se colectaron apices meristemáticos de Cymbopogon citratus L. (c) (caña santa) a partir de plantas cultivadas en invernaderos. Los ápices fueron incubados en un medio de cultivo semisólido Murashige y Skoog (MS) con 3% (m/v) de sacarosa y 0.89 µM de 6-benciladenina (BA). Los brotes fueron individualizados después de tres semanas de cultivo e inoculados en sistemas de inmersión temporal de 10 litros de capacidad, los cuales contenían 3 litros de medio de cultivo líquido de igual composición. Se estudió el efecto de tres frecuencias de inmersión (cada 12, 6 y 4 horas) en la producción de biomasa. También, se determinó el efecto de la densidad de inóculo. El crecimiento de la biomasa estuvo influenciado por la frecuencia de inmersión. La mayor proliferación de brotes (17.3 brotes/explantes), así como la mayor longitud de la planta (45.2 cm) se obtuvo en el tratamiento con una inmersión cada 4 h. La masa fresca y seca fueron también superiores en este tratamiento (153.4 gMF y 24.8 gMS, respectivamente). Después de 30 días de cultivo la máxima acumulación de biomasa se obtuvo cuando se utilizó una densidad de inóculo de 60 explantes por SIT (185.2 gMF y 35.2 gMS). Por primera vez, se logró producir biomasa de C. citratus en sistemas de inmersión temporal de 10 litros. Estos resultados representan el primer paso hacia el escalado de la producción de biomasa de esta planta medicinal en sistemas de inmersión temporal de gran capacidad.
Palabras clave:
automatización, caña santa, crecimiento de biomasa, cultivo de
tejidos, planta medicinal
Abbreviations: BA, 6-benzyladenine; MS, Murashige and Skoog basal medium; DW, dry weight; FW, fresh weight; SEM, scanning electron microscope; TIS, temporary immersion systems
INTRODUCTION
Temporary immersion system (TIS) is a cheap technology for automation of in vitro plant tissue culture and has been successful used for in vitro propagation of medicinal plants (Preil, 2005). From an economical view point, RITAÒ and TIS of 1 litre capacity has been proven to be suitable for research at laboratory scale; but for commercial application, large vessels are frequently used. Moreover, the most important tropical species are commercially propagated in TIS using twin flasks with a capacity ranging from 5 to 10 litres (Jiménez, 2005).
Because TIS allows large scale culturing of plant organs at low cost, it became an attractive alternative for the production of plant secondary metabolites. In this sense, TIS has been described for in vitro culture of a wide range of medicinal plant species such as Lavandula officinalis Chaix and Hypericum perforatum L. (Wilkenet al., 2005), Mentha spicata L. (Tisserat and Vaughn, 2008), Saccharum officinarum L. (Yang et al., 2010), Camptotheca acuminata Decne (Sankar-Thomas and Lieberei, 2011), Panax quinquefolius L. (Uchenduet al., 2011), Astragalus membranaceus (Wuet al., 2011), Digitalis lanata L. (Pérez-Alonso et al., 2012), Leocojum aestivum L. (Schumann et al., 2012).
Cymbopogon citratus (D.C.) Stapf. is a perennial and medicinal herb belonging to the Poaceae family. It has been widely cultivated in tropical and subtropical countries to produce C. citratus oil, which fundamentally contains citral, farnesol, nerol, citronellal and myrcene. Citral is an essential oil component, mainly located in the leaves and primarily used to flavour food (Paranagama et al., 2003). Although the biomass production of C. citratus has also been described by conventionally suckering (Licea et al., 1999), from callus and cell suspension (Quiala et al., 2002), only in shoots á and â citrals and free radical scavengers (Wilken et al., 2005; Tapia et al., 2007) have been detected. Moreover in previous research we reported the in vitro propagation of C. citratus in 1 liter TIS (Quiala et al., 2006). However, there are no experiences on biomass production in large culture vessels. Therefore, this research was conducted to optimize the immersion frequency and the inoculum density in 10 liters temporary immersion bioreactors as the first step in the scaling-up the biomass production of this medicinal plant in large temporary immersion bioreactors.
MATERIALS AND METHODS
Plant material and TIS culture
Shoot-tips, collected
from greenhouse-grown plants of C. citratus were surface sterilized in
a commercial solution of sodium hypochlorite (3% NaOCl) for 15 min. Under aseptic
conditions and with the help of a stereo-microscope, shoot tips of about 1.0
mm size were excised and immediately placed onto a semi-solid Murashige and
Skoog (1962) medium (MS) with 30% (w/v) sucrose, and supplemented with 0.89
µM 6-benzyladenine (BA). After three weeks of culture shoots were individualized
and used for TIS culture. Single shoots were used in all experiments; they were
inoculated in 10 litres TIS containing 3 litres of basal MS medium supplemented
with 6-benzylaminopurine (0.89 µM) and sucrose (30 g l-1).
The concept and operation of the TIS used in the experiments were based on the two-vessel system of 10 liters capacity (Nalgene®) as described by (Escalona et al., 1999). The immersion rhythm was regulated by computer software (Byosis version 1.0).
Experimental design
In the first experiment, the effect of three immersion frequencies (every 12, 6 and 4 hours each with 2 min duration) on shoot biomass growth was studied. An inoculum density of forty single shoots (8.3 gMF) per TIS was used.
In the second experiment three inoculum densities (40, 50 and 60 single shoot/TIS) corresponding to 8.8, 10.6 and 12.5 gMF respectively. Immersion frequency was selected from the results in the first experiment.
The systems were incubated at 28±2ºC under a 16/8h (day/night) photoperiod with light supplied by white fluorescent tubes (25 µmol m-2s-1). For both experiment, the number of shoots per explant, shoot length (cm), fresh weight (FW) and dry weight (DW) (g) were determined after four weeks of culture.
Statistical analysis
Data were processed using the SPSS software package for Windows ver. 15. Results were analyzed by the Non-parametric test: KruskalWallis. Differences were considered significant at p< 0.05.
RESULTS AND DISCUSSION
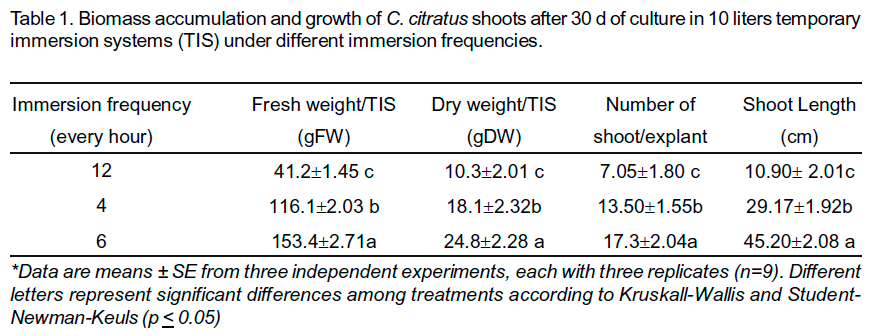
A fast increase of C. citratus biomass production in TIS was obtained in all treatments. Biomass accumulation was inûuenced by the immersion frequency. Significantly more biomass was accumulated in the shoots immersed every 4h, corresponding to six immersions per day (Table 1). Also, the highest values of dry weight, proliferation rate, as well as the plants length were obtained with this treatment.
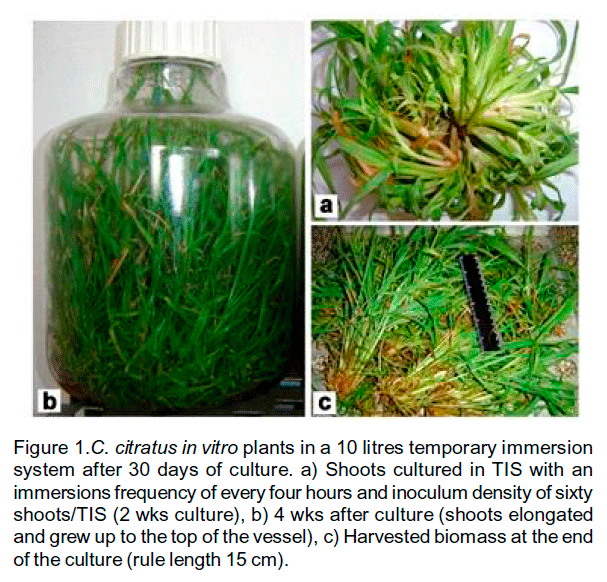
The maximum biomass and growth ratio were observed with an inoculum density of 60 explants (12.5 g of FW) (Table 2). Shoot proliferation was stimulated in the first two weeks (Figure 1a), but at the end of the experiment the shoots elongated and grew up to the top of the vessel (Figure 1b). A high amount of biomass was harvested (185.2 gFW and 35.2 gDW) after 4wks of culture (Table 2) (Figure 1c).
In an earlier study we found a major production of C. citratus biomass with four immersions per day in 1 liter TIS (Quiala et al., 2006). However, in 10 liters TIS the best results were achieved with six immersions per day. Similar results have been described by others authors. The immersion frequency had a significant effect on biomass accumulation and yield of betalains in hairy root cultures (Etienne and Berthouly, 2002). The importance of immersion frequency for the production of biomass and secondary metabolite was also demonstrated with Digitalis purpurea L. (Pérez- Alonso et al., 2009). These authors describe a positive effect of immersion frequency (every four hours) on biomass growth and cardenolides accumulation.
It was demonstrated than 10 liters TIS clearly offered an advantage for the production of a high amount of C. citratus biomass. The fast biomass growth in TIS could be the result of the combination of the advantages of both gelled culture (gas exchange) and liquid culture (increased nutrient uptake), which improves the growth of the plantlets (Etienne and Berthouly, 2002). However, renewal of the head space in the TIS with each immersion led to a higher oxygen concentration (Etienne and Berthouly, 2002), probably contributing to the higher biomass of C. citratus. In TIS, using larger vessels, large volumes of media can be used. This has a positive effect on the proliferation and growth of the shoots, after immersion a thin layer of culture medium may remain on the surface of the plant, which avoids desiccation and promotes nutrient uptake (Preil, 2005).
Figure 1.C.
citratus in vitro plants in a 10 litres temporary immersion system after
30 days of culture. a) Shoots cultured in TIS with an immersions frequency of
every four hours and inoculum density of sixty shoots/TIS (2 wks culture), b)
4 wks after culture (shoots elongated and grew up to the top of the vessel),
c) Harvested biomass at the end of the culture (rule length 15 cm).
For all TIS, the volume of the container, and hence the head space, is much
higher than in containers used for conventional procedures. Moreover, containers
ranging in size from one to twenty can usually be adapted to the system and
it is necessary to adjust some parameters that have been pre-established during
the experimental scale in smaller containers (Jiménez, 2005).
In conclusion, the optimization of the immersion frequency and the inoculum density increased the biomass growth of C. citratus in large temporary immersion bioreactors. The maximum biomass accumulation was achieved using an immersion frequency every four hours and 60 shoots/TIS per 10 liters TIS (3 liters working volume) as inoculum density. This is the first steps in the scalingup the biomass production of lemon grass in TIS. The optimization of others parameters such as the frequency of the renovation of culture medium and the optimal volume of them could be a promissory strategy for continuous improving the biomass growth of this medicinal plant in 10 liters temporary immersion bioreactors.
ACKNOWLEDGEMENTS
The authors wish
to thank the support of the EU through the ALFA Network CARIBIOTEC (project
AML/B7-311/97/ 0666/II-0201) and the
Cuban Ministry of Science, Technology and Environment (CITMA). Also to Emeritus
Prof. Dr. ir. Oswald Van Cleemput from the Faculty of Bioscience Engineering
at Gent University for the revision of the manuscript.
REFERENCES
Escalona M, Lorenzo JC, González B, Daquinta M, González JL, Desjardins Y, Borroto CG (1999) Pineapple (Ananas comosus L.Merr) micropropagation in temporary immersion systems. Plant Cell Rep 18:74374
Etienne H, Berthouly M (2002) Temporary immersion systems in plant micropropagation. Plant Cell Tiss Org Cult 69: 215231
Jiménez E (2005) Mass propagation of tropical crops in temporary immersion systems. In: Hvos- Elf T, Preil W (Eds) Liquid Culture Systems for in vitro Plants Propagation, pp.197-211. Kluwer Academic Publishers. Dordrecht.
Licea, R J, Fernández M, Alvarado K, Gómez K (1999) Influence of agar concentration on in vitro multiplication of Cymbopogon citratus (D.C.) Stapf. Biotecnología Vegetal 1(2):77-81
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473497
Paranagama P, Abeysekera T, Nugaliyadde L, Abeywickrama K (2003) Effect of the essential oils of Cymbopogon citratus, C. nardus and Cinnamomum zeylanicum on pest incidence and grain quality of rough rice (paddy) stored in an enclosed seed box. Food Agric. Environ. 2:134-136
Pérez-Alonso N, Wilken D, Gerth A, Jahn A, Nitzsche HM, Kerns G, Capote-Pérez A, Jiménez E (2009) Cardiotonic glycosides from biomass of Digitalis purpurea L. cultured in temporary immersion systems. Plant Cell Tiss and Organ Cult 99:151156
Pérez-Alonso N, Capote A, Gerth A, Jiménez E (2012) Increased cardenolides production by elicitation of Digitalis lanata shoots cultured in temporary immersion systems. Plant Cell Tiss and Organ Cult 110:153 162
Preil, W (2005) General introduction: a personal reflection on use of liquid media for in vitro culture. In: Preil, W (Ed.) Liquid culture system for in vitro plant propagation, pp. 1-18. Springer. Dordrecht
Quiala E, Barbón R, Jiménez E, de Feria M, Capote A, Pérez N, Chávez M, Bidot I (2002) Establecimiento y multiplicación de suspensiones celulares de Cymbopogon citratus (D.C) Stapf. Biotecnología Vegetal 2(3):155-161
Quiala E, Barbón R, Jiménez E, de Feria M, Capote A, Pérez N, Chávez M (2006) Biomass production of Cymbopogon citratus (D.C.) Stapf., a medicinal plant, in temporary immersion systems. In Vitro Cell Dev Biol - Plant 42: 298300
Sankar-Thomas YD, Lieberei R (2011) Camptothecin accumulation in various organ cultures of Camptotheca acuminata Decne grown in different culture systems. Plant Cell Tiss Organ Cult (2011) 106:445454
Schumann A, Berkov S, Claus D, Gerth A, Bastida J, Codina C (2012) Production of Galanthamine by Leucojum aestivum Shoots Grown in Different Bioreactor Systems. Appl Biochem Biotechnol 167: 19071920
Tapia A, Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G, Gerth A, Wilken D, Jordan M, Jiménez E, Kosky R, Quiala E (2007) Free Radical Scavengers from Cymbopogon citratus (DC.) Stapf Plants Cultivated in Bioreactors by the Temporary Immersion (TIS) Principle. Z. Naturforsch. 62: 447 - 457
Tisserat B, Vaughn SF (2008) Growth, morphogenesis, and essential oil production in Mentha spicata L. plantlets in vitro. In Vitro Cell Dev Biol Plant 44:40 50
Uchendu EE, Paliyath G, Brown DCW, Saxena PK (2011) In vitro propagation of North American ginseng (Panax quinquefolius L). In Vitro Cell Dev Biol Plant 47: 710-718
Wilken D, Jiménez E, Hohe A, Jordan M, Gómez R, Schmeda G, Gerth A (2005) Comparison of secondary metabolite production in cell suspension, callus culture and temporary immersion system. In: Hvos-Elf T, Preil W (Eds) Liquid Culture Systems for in vitro Plants Propagation, pp. 525-538. Kluwer Academic Publishers. Dordrecht
Wu SQ, Lian ML, Gao R, Park SY, Piao XC (2011) Bioreactor application on adventitious root culture of Astragalus membranaceus. In Vitro Cell Dev Biol Plant 47:719724
Yang L, Zambrano Y, Hu C, Carmona E, Bernal A, Pérez A, Zayas CM, Li YR, Guerra A, Santana I, Arencibia A (2010) Sugarcane metabolites produced in CO2-rich temporary immersion bioreactors (TIBs) induce tomato (Solanum lycopersicum) resistance against bacterial wilt (Ralstonia solanacearum). In Vitro Cellular & Developmental Biology. Plant 46(6): 558-568
Recibido: 7-01-2014
Aceptado: 28-02-2014
Copyright (c) 2016 Biotecnología Vegetal
![]()
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.