Artículo original
Biotecnología Vegetal Vol. 19, No. 3: 205 - 213, julio - septiembre, 2019
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Maltose in culture media improves the in vitro regeneration of Urochloa brizantha cv. ‘Marandu’ plants
La maltosa en los medios de cultivo mejora la regeneración in vitro de plantas de Urochloa brizantha cv. ‘Marandu’
Alyne Valéria Carrion Pereira1, Luciana Midori Takamori1, Diliane Harumi Yaguinuma1, Adriana Mendonça de Oliveira1, Alessandra Ferreira Ribas1*
1 Laboratório de Cultura de Tecidos Vegetais, Universidade do Oeste Paulista. Rodovia Raposo Tavares km 572. Limoeiro. Presidente Prudente. Brasil. 19067 175.
*Author for correspondence e-mail: alessandra_ribas@hotmail.com
ABSTRACT
The improvement of the in vitro regeneration of Urochloa brizantha is an important step to toward the development of transgenic cultivars. The objective of this work was to test the effect of the amino acid proline and the replacement of sucrose by maltose as carbon source in culture media to optimize the somatic embryogenesis. Mature seeds of U. brizantha cv. ‘Marandu’ were used as initial explant. The seeds were scarified in sulfuric acid, manually peeled disinfested and washed. The callus induction media were composed basically by Murashige and Skoog salts supplemented with 30 g l-1 sucrose, 3 mg l-1 2,4-dichlorophenoxy acetic acid, 300 mg l-1 hydrolyzed casein and 8 g l-1 agar. Carbon sources maltose or sucrose at 30, 40 and 50 g l-1 was added in the callus induction medium. In another experiment the L-proline was added to the media at 100, 200 and 400 mg l-1 . It was evaluated the number of seeds that produced callus, calluses growth after 14 weeks (mg) and number of regenerated plants. The results showed maltose was more efficient than sucrose for regenerate the plants. Nevertheless, the addition of proline to the media not improved it. Therefore, substitution of sucrose by maltose to the in vitro culture media of U. brizantha increase the plant regeneration.
Keywords: brachiaria, carbon source, somatic embryogenesis
RESUMEN
La mejora de la regeneración in vitro de Urochloa brizantha es un paso importante hacia el desarrollo de cultivares transgénicos. El objetivo de este trabajo fue evaluar el efecto del aminoácido prolina y el reemplazo de sacarosa por maltosa como fuente de carbono en medios de cultivo para optimizar la embriogénesis somática. Semillas maduras de U. brizantha cv. ‘Marandu’ fueron utilizados como explante inicial. Las semillas fueron escarificadas en ácido sulfúrico, peladas manualmente, desinfectadas y lavadas. Los medios de cultivo para la formación de callos estuvieron compuestos básicamente por sales de Murashige y Skoog con 30 g l-1 de sacarosa, 3 mg l-1 de ácido 2,4-diclorofenoxi acético, 300 mg l-1 de caseína hidrolizada y 8 g l-1 de agar. Se añadieron fuentes de carbono maltosa o sacarosa a 30, 40 y 50 g l-1 en el medio de cultivo de formación de callos. En otro experimento, se añadió L-prolina a los medios de cultivo a 100, 200 y 400 mg l-1 . Se evaluó el número de semillas que produjeron callos, el crecimiento de callos después de 14 semanas (mg) y el número de plantas regeneradas. Los resultados mostraron que la maltosa era más eficiente que la sacarosa para regenerar las plantas. La adición de prolina a los medios de cultivo no lo mejoró. Por lo tanto, la sustitución de sacarosa por maltosa en los medios de cultivo in vitro de U. brizantha aumenta la regeneración de las plantas.
Palabras clave : braquiaria, fuente de carbono, embriogénesis somática
INTRODUCTION
Native from Africa, the Urochloa (Beauv.) genus belongs to Poaceae family and has approximately 100 species (Soreng et al., 2017) that are among the most important for the world livestock. U. brizantha popularly known as brachiaria grass is one of the main forage grass to feed cattle in Brazil and feed a herd of 218.23 million head of cattle (IBGE, 2016).
The improvement of in vitro regeneration system in this grass is an important biotechnological approach aiming to produce transgenic varieties. Until now, no commercial transgenic plant of Urochloa brizantha was produced. In this way, an efficient regeneration system is the first requirement to obtaining such plants. Some modifications on the basic culture media has shown to increase the induction and regeneration efficiency of in vitro plants. For example, the addition of the amino acid L-proline and the replacement of sucrose by maltose as carbon sources have improve cereal regeneration and transformation (Hiei et al., 2014). Although sucrose is the most widely used carbon source in tissue culture, the replacement by maltose showed better results in somatic embryogenesis and plant regeneration for a number of species including: indica rice (Oryza sativa L.) (Joyia and Khan, 2013), Pinus (Pullman and Bucalo, 2014), Gossypium spp. ( Juturu et al., 2015; Kumar et al., 2015), and sugarcane (Saccharum officinarum L.) ( Kaur and Kapoor, 2016), in different cultivars of Triticum aestivum L. (Ren et al., 2010; Malik et al., 2017) and lemongrass (Cymbopogon schoenanthus L.), a medicinal plant (Abdelsalam et al., 2018).
The addition of L-proline in the media for in vitro plants regeneration has also demonstrated to improve somatic embryogenesis in different plants such as triticale a hybrid of wheat (Triticum ), rye (Secale ) and wheat (Triticum aestivum L.) (Asif et al., 2013), Persea americana Mill. (Encina et al., 2014), big bluestem (Andropogon gerardi Vitman.) (Pantha et al., 2016), sugarcane (Kaur and Kapoor, 2016).
The protocols published for in vitro regeneration of Brachiaria grass so far only used sucrose as carbon source (Takamori et al., 2015; Cabral et al., 2018; Yaguinuma et al., 2018). However, the replacement of sucrose by maltose and the addition of different concentrations of L-proline to the culture media was never be assayed for brachiaria in vitro plants regeneration. According to that, the aim of this paper was to test the effect of the amino acid proline and the replacement of sucrose by maltose as carbon source in culture media to optimize the somatic embryogenesis of U. brizantha cv. ‘Marandu’ .
MATERIAL AND METHODS
Plant material and callus induction medium
Mature seeds of U. brizantha cv. ‘Marandu’ were used as initial explants for calluses induction as described in Takamori et al. (2015). The seeds were scarified by immersion in concentrated sulfuric acid (98%) in a beaker and mixed with a glass stick for 15 minutes. The seeds were washed in tap water to remove the acid and it were dried at room temperature. Scarified seeds were manually peeled and disinfested by immersion in 70% (v/v) ethanol for 5 minutes and in 5% (v/v) sodium hypochlorite containing three drops of Tween 80® for 20 minutes followed by five washes in sterile bidistilled water.
The calluses induction medium (CIM) was composed by MS salts (Murashige and Skoog, 1962) supplemented with 3 mg l-1 2,4-dichlorophenoxy acetic acid (2,4-D) and 300 mg l-1 casein hydrolyzed, and solidified with 8 g l-1 agar. The pH of the medium was adjusted to 5.8 ± 0.1 and it was sterilized by autoclave for 20 minutes at 121 ±1 °C. As carbon source maltose or sucrose at 30, 40 and 50 g l-1 were added to the CIM medium. The calluses were sub-cultured every 14 days under the same conditions and kept in the dark (26±2 °C). In the last sub-culture it remained for 30 days under these conditions. Thereafter, the calluses with embryogenics structures were subcultured to regeneration medium with half-strength MS (MS/2), 2 mg l-1 6- benzylaminopurine (BAP) and the same concentration of each carbon source tested.
In another experiment, the amino acid L-proline was added to the CIM medium at 100, 200 and 400 mg l-1 , as described above, and containing 30 g l-1 sucrose.
For both, (carbon sources and proline), ten seeds were initially placed in each Petri dish containing CIM medium and the respective concentration to be tested (proline or carbon sources). After 28 days, the number of seeds that produced primary calluses were recorded and the percentage was calculated.
Six pro-embryogenic calluses per treatment were used to evaluate their growth as fresh mass (mg) at 14 weeks after inoculation. For regeneration the in vitro plantlets, the callus were transferred to light (30 µmol m-2 s-1) at 26±2 °C. The number of regenerated plants were recorded when plants had at least 5 cm in high and had a well-developed roots.
Double staining with acetocarmine and Evans blue was used to distinguish embryogenic from non-embryogenic callus as described in Takamori et al. (2015).
Statistical analysis
The experimental design was completely randomized with ten replicates, each repetition consisting of one Petri dish with six calluses each. The data were normalized and subjected to analysis of variance (ANOVA) to detect the significant differences. The mean separation was conducted by Fisher's Least Significance Difference (LSD) (P<0.05) using SISVAR Software Version 5.3 (Ferreira, 2011).
RESULTS AND DISCUSSION
Highly efficient in vitro regenerations protocols for Urochloa brizantha is a fundamental step to develop transgenic plants. Calluses were induced from mature seeds of U. brizantha (Figure 1 A). Double staining showed embryogenic (colored in red) from non-embryogenic callus (colored in blue) (Figure 1 B). This staining have been successfully used in different species to indicate embryogenic cells (Ahn et al., 2017; Varis et al., 2018). U. brizantha calluses friable with cream color were highly embryogenic and regenerated morphogenetic normal plants.

Figure 1. Urochloa brizantha cv. ‘Marandu’ callus (A). Double stained callus with evans blue and Acetocarmine dyes, 40x magnification. Bars 1 cm (A) and 500 µm (B).
In this work, calluses of U. brizantha in media supplemented with sucrose showed no difference in primary induction calluses for any concentration (Table 1). The callus growth was damage after 14 weeks in the culture media with sucrose at 50 g l-1 (Table 1). Plant regeneration was higher with 30 g l-1 sucrose (Figure 2 A, Table 1) while at 40 and 50 g l-1 the number of plantlets was progressively reduced (Figure 2 BC, Table 1). At these concentrations there was almost no seedlings (Figure 3 A, Table 1). Due to the heterotrophic condition of the in vitro plant culture, the addition of a carbon source is essential in the medium to develop embryogenic calluses. Different kinds and concentrations of sugars in the culture media can change the osmotic pressure and modify the morphological response of the in vitro plant tissues (Joyia and Khan, 2013). This occurs probably due to their differential role in vascular differentiation, metabolism, endogenous content of sugars in cultured tissues, and differential sensitivity (Yaseen et al., 2103).
In media supplemented with maltose there were no differences in induction and growth of calluses in media with different concentrations (Table 1). However, maltose at 40 and 50 g l-1 produced a higher number of regenerated plants (Table 1, Figure 1) increasing progressively according to the maltose concentration (Figure 2 DEF) (Table 1, Figure 3 B). The average number of regenerated plants at 50 g l-1, was almost three times greater than the number of plants regenerated with 30 g l-1 sucrose, usually used in the MS medium (Table 1). These data indicate that concentrations of 40 and 50 g l-1 maltose are beneficial to the regeneration of plants in U. brizantha.
The results demonstrated that the replacement of the sucrose by maltose in culture media improved the number of regenerated plants. In other species, like conifers, it was suggested that during the course of somatic embryogenesis process, specific media composition can be necessary for each phase because the alteration on water potential in the media affect the embryo development (Shimomae et al., 2013; Pullman and Bucalo, 2014).
Brachiaria was able to form callus in both maltose and sucrose although plant regeneration was greatly affected by the source of carbon used. Whereas maltose at 40 and 50 g l-1 regenerated the greater number of plant the same concentration of sucrose inhibited plant regeneration. Sucrose hydrolysis more rapidly leading cells to storage compounds and fast proliferation whereas maltose has slow hydrolysis and this could be a biochemical signal to induce somatic embryos development (Yaseen et al., 2103) . In kodo millet (Paspalum scorbiculatum Linn.) plants, maltose compared with others carbon source (glucose, fructose and sucrose) yield both great number of somatic embryos and rapid elongation of shoots for plantlet conversion (Ceasar and Ignacimuthu, 2010). The rapid stimuli of cell differentiation and metabolism provided by maltose generating readily available energy for a longer period as compared to other carbon sources (Kaur and Kapoor, 2016), it could be the reason for this result.
Besides that, maltose in the culture media avoid browning of cells during embryogenesis leading to an improvement of embryogenic calluses formation, somatic embryo germination and shoot length for cotton (Gossypium spp.) (Juturu et al., 2015). The slower rate of extracellular hydrolysis of maltose compared to sucrose result in less phenolic secretion into culture medium and this thought to be a reason for the best performance of maltose face to sucrose (Kumar et al., 2015). Although phenolic segregation was not observed in U. brizantha for both carbon sources, maltose in culture media improved the plant regeneration compared to sucrose.
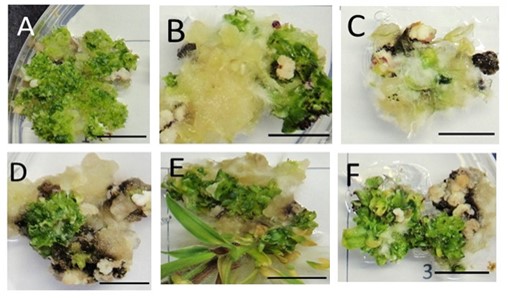
Figure 2. Urochloa brizantha cv. ‘Marandu’ callus morphology cultivated in media supplemented with different concentrations of carbon sources. ABC sucrose and DEF maltose. AD 30 g l-1 , BE 40 g l-1 and EF 50 g l-1 , respectively. Bars 1 cm.

Figure 3. Regenerated plants of Urochloa brizantha cv. ‘Marandu’ grown in media supplemented with different concentration of sucrose or maltose as carbon sources. A: sucrose, B: maltose, 1, 2 and 3 = 30, 40 and 50 g l-1 , respectively.
Table 1. Calluses of Urochloa brizantha cv. ‘Marandu’ grown in media with maltose or sucrose as carbon sources.

The addition of different concentrations of amino acid L-proline in the CIM medium for inducing brachiaria plants regeneration showed less favorable results. Initially, the addition of L-proline to the media do not favored the percentage of seed that produced primary calluses (Table 2). L-proline at 100 mg l-1 in the media promoted calluses growth after 14 weeks cultivation and did not differed from other concentrations tested. Although there was increase in calluses weight it not reflected in the average number of regenerated plants (Table 2, Figure 4).
Table 2. Urochloa brizantha cv. ‘Marandu’ calluses grown and plant regeneration on media with L-proline concentrations.
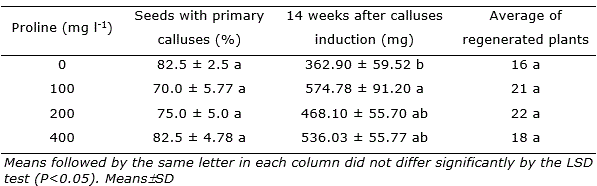

Figure 4 . Urochloa brizantha cv. ‘Marandu’ callus morphology cultivated in media supplemented with different concentrations of L-proline. (A) without proline addition, (B) 100 mg l-1 , (C) 200 mg l-1 , (D) 400 mg l-1 L-proline and regeneration of plants at the eighteenth week of culture at the same concentrations, respectively. (1) Without proline addition, (2) 100 mg l-1 , (3) 200 mg l-1 , (4) 400 mg l-1 .
The amino acid L-proline when added to the culture media function as an elicitor and stimulate the pentose phosphate pathway that directs toward the shikimate and phenylpropanoid pathway, leading to the alteration on secondary metabolism (Dias et al., 2016). It takes part in proteins synthesis and in the regulation of very important functions like osmotic adjustment and protection of proteins during stress conditions. It is believed that it participate in proteins structure because deficiency in proline biosynthesis leads to abnormal plants and cell wall defects (Kishor et al., 2015). In addition to in vitro osmoprotection, proline has multiple functions such as protein stabilization, inhibition of protein aggregation and reduction the ROS levels under oxidative stress conditions (Bach and Takagi, 2013).
In this work, the regeneration of brachiaria was morphological normal even in the absence of proline in culture media. Average number of regenerated plants was not significant when compared to the ones regenerated in media without proline. This fact could be due to the concentration used. The highest concentration tested was 400 mg l-1 while for regeneration of the grass Andropogon gerardii, Pantha et al. (2016) added 2 g l-1 of proline. On the other hand, for sweet sorghum (Sorghum bicolor L.) the addition of proline (range from 0 – 2.4 g l-1 ) was not necessary for the calluses induction (Zhao et al., 2010). Like sorghum, the in vitro regeneration of brachiaria grass was not improved by the addition of low proline concentration (up to 400 mg l-1). Higher concentrations of proline may be will test in the regeneration media.
CONCLUSIONS
Maltose replacing sucrose in culture media for somatic embryogenesis of brachiaria improves the number of in vitro regenerated plants while the addition of L-proline up to 400 mg l-1 did not.
Conflicts of interest
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This study was supported by project no. 2013/04819-4 from São Paulo Research Foundation (FAPESP). D.H.Y was supported by IC grant no. 2016/06874-0, A.M.O. and A.V.C.P received a TT3 fellowship numbers 2015/14352-1 and 2014/07830-1, respectively from São Paulo Research Foundation (FAPESP). L.M.T was supported by a scholarship from Coordination for the Improvement of Higher Education Personnel (CAPES) code 001.
REFERENCES
Abdelsalam A, Chowdhury K, El-Bakry A (2018) Efficient adventitious morphogenesis from in vitro Cultures of the medicinal plant Cymbopogon schoenanthus. Plant tissue Cult & Biotechnology 28(2): 147-160; doi:10.3329/ptcb.v28i2.39674
Ahn CH, Tull AR, Montello PM, Merkle SA (2017) A clonal propagation system for Atlantic white cedar (Chamaecyparis thyoides) via somatic embryogenesis without the use of plant growth regulators. Plant Cell Tissue Organ Culture 130: 91-101; doi:10.1007/s11240-017-1206-7
Asif M, Eudes F, Goyal A, Amundsen E, Randhawa H, Spanner D (2013) Organelle antioxidants improve microspore embryogenesis in wheat and triticale. In vitro Cell Development Biology Plant 49: 489-497; doi:10.1007/s11627-013-9514-z
Bach TMH, Takagi H (2013) Properties, metabolisms, and applications of L-proline analogues. Applied Microbiology Biotechnology 97: 6623-6634; doi:10.1007/s00253-013-5022-7
Cabral GB, Carneiro VTC, Gomes ACMM, Lacerda AL, Martinelli AP, Dusi DMA (2018) Genetic transformation of Brachiaria brizantha cv. ‘Marandu’ by biolistics. Anais da Academia Brasileira de Ciências 90(2): 1789-1797; doi:10.1590/0001-3765201820170842
Ceasar SA, Ignacimuthu S (2010) Effects of cytokinins, carbohydrates and amino acids on induction and maturation of somatic embryos in kodo millet (Paspalum scorbiculatum Linn.). Plant cell, tissue and organ culture 102(2): 153-162; doi:10.1007/s11240-010-9716-6
Dias MI, Sousa MJ, Alves RC, Ferreira ICFR (2016) Exploring plant tissue culture to improve the production of phenolic compounds: A review. Industrial Crops and Products 82: 9-22; doi:10.1016/j.indcrop.2015.12.016
Encina CL, Parisi A, O’Brien C, Mitter N (2014) Enhancing somatic embryogenesis in avocado (Persea americana Mill.) using a two-step culture system and including glutamine in the culture medium. Scientia Horticulturae 165: 44-50; doi:10.1016/j.scienta.2013.10.019
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia 35(6): 1039-1042; doi:10.1590/S1413-70542011000600001
Hiei Y, Ishida Y, Komari T (2014) Progress of cereal transformation technology mediated by Agrobacterium tumefaciens. Frontiers in Plant Science 7(5): 628; doi:10.3389/fpls.2014.00628
IBGE (2016) Instituto Brasileiro de Geografia e Estatística Produção da pecuária municipal. Available in: https://biblioteca.ibge.gov.br/visualização/periodicos/84/ppm_2016_v44_br.pdf. Accessed 30/09/2018
Joyia FA, Khan MS (2013) Scutellum-derived callus-based efficient and reproducible regeneration system for elite varieties of indica rice in Pakistan. International Journal of Agriculture & Biology 15(1): 27-33
Juturu VN, Mekala GK, Kirti PB (2015) Current status of tissue culture and genetic transformation research in cotton (Gossypium spp.) Plant cell tissue Organ Culture 120: 813-839; doi: 10.1007/s11240-014-0640-z
Kaur R, Kapoor M (2016) Plant regeneration through somatic embryogenesis in sugarcane. Sugar Tech 18(1): 93-99; doi: 10.1007/s12355-015-0380-3
Kishor PBK, Kumari PH, Sunita MS, Sreenivasulu N (2015) Role of proline in cell wall synthesis and plant development and its implications in plant. Frontiers in Plant Science 20(6): 544; doi:10.3389/fpls.2015.00544
Kumar GP, Subiramani S, Govindarajan S, Sadasivam V, Manickam V, Mogilicherla K, Kumas S, Thiruppathi K, Narayanasamy J (2015) Evaluation of different carbon sources for high frequency callus culture with reduced phenolic secretion in cotton (Gossypium hirsutum L.) cv. Svpr-2. Biotechnology Reports 7: 72-80; doi:10.1016/j.btre.2015.05.005
Malik K, Birla D, Yadav H, Sainger M, Chaudhary D, Jaiwal PK (2017) Evaluation of carbon sources, gelling agents, growth hormones and additives for efficient callus induction and plant regeneration in indian wheat (Triticum aestivum L.) genotypes using mature embryos. Journal of Crop Science Biotechnology 20: 185-192; doi:10.1007/s12892-017-0046-0
Murashige T, Skoog FA (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15(3): 473-497; doi: 10.1111/j.1399-3054.1962.tb08052.x
Pantha P, Ponniah SK, Ntamatungiro S, Manoharan M (2016) Improved embryogenic callus induction and plant regeneration in big bluestem (Andropogon gerardii Vitman), a potential bioenergy feedstock. African Journal of Biotechnology 15(39): 2166-2171; doi: 10.5897/ajb2016.15542
Pullman GS, Bucalo K (2014) Pine somatic embryogenesis: analyses of seed tissue and medium to improve protocol development. New Forest 45: 353–377; doi:10.1007/s11056-14-9407-y
Ren JP, Wang XG, Jun YIN (2010) Dicamba and sugar effects on callus induction and plant regeneration from mature embryo culture of wheat. Agricultural Sciences in China 9(1): 31-37; doi: 10.1016/S1671-2927(09)60064-X
Shimomae K, Chin DP, Khan RS, Mii M (2013) Efficient plant regeneration system from seed-derived callus of ravenna grass (Erianthus ravennae (L.) Beauv.). Plant biotechnology 30(5): 473-478; doi:10.5511/plantbiotechnology.13.0721a
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Teisher JK, Clark LG, Barbera P, Gillespie LJ, Zuloaga FO (2017) A worldwide phylogenetic classification of the Poaceae (Gramineae) ii: An update and a comparison of two 2015 classifications. Journal of Systematics and Evolution 55(4): 259-290; doi: 10.1111/jse.12262
Takamori LM, Machado Neto NB, Vieira LGE, Ribas AF (2015) Optimization of somatic embryogenesis and in vitro plant regeneration of Urochloa species using Picloram. In Vitro Cell Developmental Biology Plant 51(5): 554-563; doi:10.1007/s11627-015-9701-1
Varis S, Krystyna Klimaszewska K, Aronen T (2018) Somatic embryogenesis and plant regeneration from primordial shoot explants of Picea abies (L.) H. Karst. somatic trees Frontiers in Plant Science 9: 1551; doi:10.3389/fpls.2018.01551
Yaguinuma DH, Takamori LM, Oliveira AM, Vieira LGE, Ribas AF (2018) In vitro regeneration from leaf-base segments in three genotypes of Urochloa spp. Crop & Pasture Science 69: 527-534; doi:10.1071/CP17395
Zhao L, Liu S, Song S (2010) Optimization of callus induction and plant regeneration from germinating seeds of sweet sorghum (Sorghum bicolor Moench). African Journal of Biotechnology 9(16): 2367-2374
Recibido: 19-04-2019
Aceptado: 12-06-2019
Copyright (c) 2019 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
