Artículo de revisión
Biotecnología Vegetal Vol. 20, No. 1: 01-16, enero-marzo, 2020
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Potential of Trichoderma species as biofertilizer and biological control on Oryza sativa L. cultivation
Potencial de especies de Trichoderma como biofertilizante y control biológico en el cultivo de Oryza sativa L.
Sanjit Debnath1* https://orcid.org/0000-0001-8896-8128
Goutam Chakraborty1 https://orcid.org/0000-0003-4879-8376
Suvendhu S Dutta1 https://orcid.org/0000-0003-2726-9088
Shaon Ray Chaudhuri2 https://orcid.org/0000-0001-6277-4798
Panna Das3
Ajay Krishna Saha1 https://orcid.org/0000-0003-4423-8216
1Mycology and Plant Pathology Laboratory, Department of Botany, Tripura University. Suryamaninagar. Tripura. India.
2Department of Microbiology, Tripura University. Suryamaninagar. Tripura. India.
3Microbiology Laboratory, Department of Botany, Tripura University. Suryamaninagar. Tripura. India.
*Author for correspondence e-mail: sanjitdebnath2888@gmail.com
ABSTRACT
Rice (Oryza sativa L.) is one of the main staples around the world, especially in Asia, the most consuming region. Rice cultivation currently faces many environmental problems caused by the contribution of chemical fertilizers and pesticides. This review is carried out to document the principal findings of Trichoderma species application on rice plants. The analysis revealed that different species of Trichoderma have been applied in different fields of research on rice plants to increase the absorption of nutrients, soil fertility, tolerance to salinity, plant growth promotion and development with increased yields. Mechanisms include decomposition, mycoparasitism, cellulose degradation, and phosphate solubilizing activity. The potential for antagonistic activity against disease-causing fungi and inhibition of growth of harmful root microflora is demonstrated. This work also suggested that Trichoderma can be applied alone or together with other microorganisms to significantly promote the growth and performance of rice plants. Among the species, T. harzianum was studied mainly to determine its different roles in rice, followed by T. asperellum, T. viride, T. virens and T. atroviride. Therefore, the findings revealed the potential of the Trichoderma species as a possible biofertilizer and biological control for rice cultivation worldwide.
Keywords: rice, biocontrol agent, growth parameters and yield
RESUMEN
El arroz (Oryza sativa L.) es uno de los principales alimentos básicos en todo el mundo, especialmente en Asia, la región más consumidora. El cultivo de arroz en la actualidad enfrenta muchos problemas ambientales causados por el aporte de fertilizantes químicos y plaguicidas. El objetivo de este trabajo de revisión fue documentar los principales hallazgos sobre el empleo de especies de Trichoderma en el cultivo de arroz. Este análisis reveló que diferentes especies de Trichoderma se han aplicado en diferentes campos de investigación sobre plantas de arroz para aumentar la absorción de nutrientes, la fertilidad del suelo, la tolerancia a la salinidad, promoción del crecimiento y desarrollo de las plantas con incremento en los rendimientos. Entre los mecanismos se menciona la descomposición, el micoparasitismo, la degradación de celulosa y la actividad solubilizadora de fosfatos. Se demuestra el potencial de actividad antagonista contra hongos causantes de enfermedades y la inhibición del crecimiento de la microflora perjudicial de la raíz. Este trabajo también sugirió que Trichoderma puede aplicarse solo o junto con otros microorganismos para promover significativamente el crecimiento y el rendimiento de las plantas de arroz. Entre las especies, T. harzianum se estudió principalmente para determinar sus diferentes roles en el arroz, seguido de T. asperellum, T. viride, T. virens y T. atroviride. Por lo tanto, los hallazgos revelaron el potencial de las especies de Trichoderma como un posible biofertilizante y control biológico para el cultivo de arroz en todo el mundo.
Palabras clave: arroz, agente de biocontrol, parámetros de crecimiento y rendimiento
INTRODUCTION
Among the major food crops, rice (Oryza sativa L.) serves as the staple food in terms of area under cultivation, production and consumer preference catering to more than half of the population of the world including two third of Indian population (Bishwajit et al., 2013; Huang et al., 2014).
India is the second largest producer and patron of rice production in the international level (Singh et al., 2018). However, the production of this crop is progressively restricted by varied environmental stresses in Asia (Dar et al., 2013).
Similarly, world rice production is significantly reduced by drought because of unpredictable and uneven rain throughout the growing season (Crosson, 1995). Therefore, the crises of food demands for improved farming practices resulting in enhanced rice production, particularly in areas that are highly susceptible to varied types of environmental stresses (Singh and Singh et al., 2017).
Due to massive application of chemical fertilizers, it directly or indirectly causes different environmental pollutions (Khan, 2018). The use of chemical fertilizers and pesticides lead to system damage through emergence of resistant pathogens as well as soil toxicity due to persistent heavy metals (Habibah et al., 2011; Doni et al., 2014a). Hence, there is a need to promote the use of biofertilizers that are environment friendly (Banayo et al., 2012).
Chemical fertilizer is harmful to living beings and specially, adversely affects the microbial population present in the ecosystems (Khan and Singh, 2015). It is therefore essential to develop an integrated nutritional support policy to supplement and build smart fertilizer application with the addition of adequate eco-friendly resources. Utilization of microorganisms has been hypothesized to play vital role in mitigating stress and thereby enhancing yields in rice (Man and Ha, 2006; Zaidi et al., 2018).
Besides, beneficial microorganisms have been reported to be involved in maintaining agricultural production, protecting the ecosystem and decreasing the use of chemical fertilizers (Adesemoye and Kloepper, 2009). For instance, soil bacteria such us Pseudomonas and Azospirilium are known to alleviate abiotic stresses in numerous crop species including rice (Vejan et al., 2016).
On the other hand, Trichoderma is amongst the foremost extensively researched microbes and is primarily recognized for its mycoparasitic activities and also helps plants to reduce stress through induction of morphological, physiological and organic changes (Ji et al., 2012; Mukherjee et al., 2013; Doni et al., 2014d). The first description of Trichoderma dates back to 1794 (Person, 1794) and these ubiquitous fungi have tremendous impact on human life and ecosystem functionality (Felix et al., 2014).
Trichoderma are one such free-living fungi which are highly interactive in root, soil and foliar environments (Harman et al., 2004) and are available to natural growers as large variety of Trichoderma-based biopesticides that may be useful for the management of plant diseases (Singh et al., 2014). It is also reported that Trichoderma enhance root growth, promotes plant growth, improves seedling vigour, promotes plant development, increases absorption of nutrients, helps seed germination under adverse soil conditions, imparts tolerance to abiotic stresses, secretes phosphate-solubilizing enzymes and phytohormones, improves yield parameters and represses growth of harmful root microflora (Harman et al., 2004; Shoresh and Harman, 2008; Mastouri et al., 2010; Shukla et al., 2012; Hermosa et al., 2012; Chen et al., 2013; Pandey et al., 2016; Swain et al., 2018).
Besides, Trichoderma species (T. virens, T. viride, T. harzianum) are involved in decomposition, mycoparasitism and even in polyose degradation (Samuels, 1996; Harman et al., 2004; Druzhinina et al., 2006; Jiang et al., 2011).
In addition, some Trichoderma species (T. harzianum, T. asperellum, T. atroviride, T. gamsii, T. hamatum, T. koningii, T. lignorum, T. parceanamosum, T. polysporum, T. virens and T. viride) are well recognized as biofungicides in agricultural practices (Verma et al., 2007; Woo et al., 2014). Integrated disease management by using biocontrol agents (BCAs) is precious to sustainable agriculture and it helps to reduce crop losses caused by plant pathogens using natural mechanisms involving numerous modes of action including antibiosis, mycoparasitism, antagonism and induced resistance. Scientists are striving to identify potential strains of BCA that upon combination may result in a synergistic response. Much of the published work claiming synergy showed antagonistic relations among BCAs (Xu et al., 2011).
In rice cultivation several papers reports the use of Trichoderma species with different applications. For this reason, the present review is carried out to document the principal findings of Trichoderma species application on rice plants.
Effect of Trichoderma on yield enhancement of rice
Different species of Trichoderma showed beneficial effects on soil application (Cuevas et al., 1995; Cuevas et al., 2001; Cuevas et al., 2005; Cuevas, 2006) (Table 1, Table 2, Table 3). Doni et al. (2017) reported that System of Rice Intensification (SRI) with the application of T. asperellum showed yield components with highest panicle number, spikelets per panicle and more filled grains on rice plants as compared to other treatments. These effects were established by measuring the physiological and growth parameters.
Table 1. Effect of Trichoderma spp. strains applied in Oryza sativa L. cultivation.
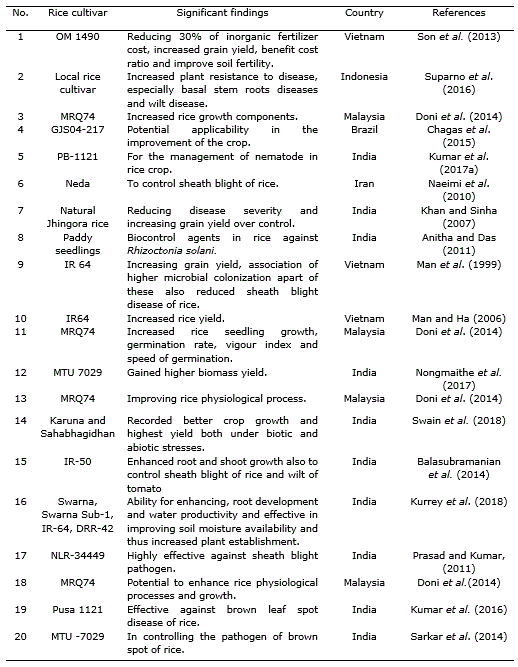
Table 2. Effect of Trichoderma harzianum strains applied in Oryza sativa L. cultivation.
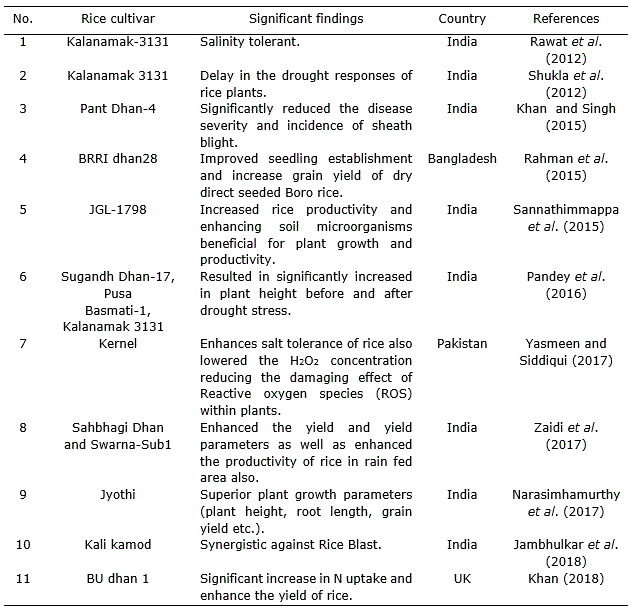
Table 3. Effect of different Trichoderma species strains applied in Oryza sativa L. cultivation.
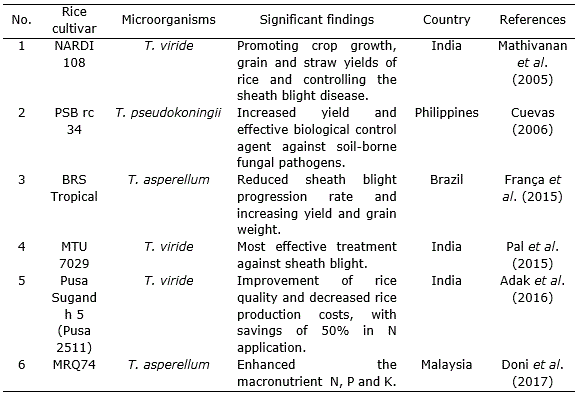
In this sense, Khadka and Uphoff (2019) described many benefits of Trichoderma inoculation for improving crop production which includes growth and yield enhancement and the alleviation of biotic and abiotic stresses. They also evaluated the effects of Trichoderma inoculation of rice plants under SRI management compared with transplanted and flooded rice plants and the results indicated significantly better performance (P=0.01) associated with Trichoderma inoculation in terms of grain yield and other growth-contributing factors, compared to non-inoculated rice cropping.
Trichoderma promotes growth, increases rice grain as well as vegetable yield even with minimal mineral fertilizer application due to better mineral accessibility (especially P and Zn) to plants. For example, sowing of seed after osmopriming with 3% ZnSO4 and biopriming with Trichoderma and then application of sulphur fungicide at 20 days after sowing documented for improving seedling establishment and increasing grain yield of Boro rice (Rahman et al., 2015).
Another example is the application of Trichoderma species with composted rice straw in conjunction with 70% NPK. It was found to be economically efficient i.e., benefit cost ratio was highest among all treatments. The nutrient content (organic carbon, available N, P and exchangeable K) in the rice soil was improved significantly with Trichoderma as compared to the other conventional application by the dose of farmers (burnt rice straw with 100% NPK alone). The combination of Trichoderma with 70% NPK and burnt rice straw reduced inorganic fertilizer cost while increasing grain yield, benefit cost ratio and soil fertility (Son et al., 2013). Application of the combination of T. harzianum with NPK treatments efficiently worked for conservation of soil nutrients under rice ecosystem through proper decomposing method, concurrently enhancing soil microorganisms that are beneficial for plant growth and productivity (Man et al., 1999; Sannathimmappa et al., 2015).
Antagonistic effect of Trichoderma in rice disease management
Trichoderma species has been shown antagonism effect on the causal agents of several diseases in rice in vitro and in field assays. Trichoderma have long been recognized as biocontrol agents are highly interactive in root, soil and foliar environments and produce a variety of compounds that induce localized and systemic resistance responses in plants (Table 1, Table 2, Table 3, Table 4).
Table 4. Effect of Trichoderma strains combined with other microorganism in Oryza sativa L. cultivation.
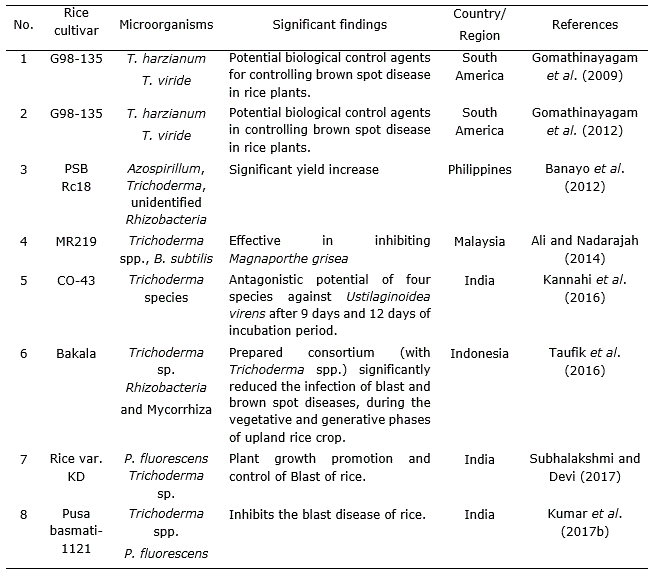
Gomathinayagam et al. (2009) suggested that T. viride has a potent antagonistic activity against the pathogen which causes brown spot disease in rice (Cochliobolus miyabeanus). Trichoderma spp. are able to increase plant resistance to disease, especially in case of basal stem rot disease (Sclerotium oryzae) as well as wilt disease (Xanthomonas sp.) and it also plays a significant role as decomposer to provide plant nutrients (Man and Ha, 2006; Suparno et al., 2016). Consortium of Rhizobacteria and mycorrhiza with Trichoderma spp. notably reduced the infection of blast (Magnaporthe grisea Herbert) and brown spot disease (C. miyabeanus) in upland rice crops (Taufik et al., 2016; Kumar et al., 2016).
It is also an effective biological control agent against soil-borne fungal pathogens. Trichoderma spp. increases resistance against Bakanae disease of MR219 rice by increasing resistance to the pathogen Fusarium fujikuroi (Ng et al., 2015). Rice blast disease caused yield losses throughout the world but Trichoderma individually or in mixed inoculums with Bacillus subtilis Cohn gave the best reduction in pre-post damping off disease infection caused by rice blast fungus M. grisea (Ali and Nadarajah, 2014). Combined application of Pseudomonas fluorescens Migula and Trichoderma species showed significant role in plant growth enhancement and management of blast of rice (Subhalakshmi and Devi, 2017; Jambhulkar et al., 2018).
T. harzanium and T. viride are potential biological control agents and there are significant differences between these two fungi in controlling brown spot disease (C. miyabeanus) in rice plants (Gomathinayagam et al., 2012). Trichoderma spp. isolates found in the commercial product Trichoplus JCO were capable of solubilizing phosphate in substance, suggesting its potential pertinence in the improvement of the crop (Chagas et al., 2015). Kannahi et al. (2016) studied the efficacy of various isolates of T. viride, T. virens, T. harzianum and T. reesei against fungus by dual culture methodology under various in vitro conditions and found that each one of the isolates of Trichoderma species showed antagonistic activity. Kumar et al. (2017a) found that Trichoderma can be applied to crops with agrochemicals for controlling of root-kont nematode (Meloidogyne graminicola). França et al. (2015) demonstrated that mixed isolates of T. asperellum used during seed treatment and foliar spray efficiently increased yield and grain weight in cultivated rice under flooded conditions while minimizing the incidence of rice sheath blight (Rhizoctonia solani J.G.Kühn).
Numerous researchers reported that different Trichoderma sp. like, T. harzianum (Khan and Sinha, 2007; Shahram et al., 2010), Trichoderma sp. (Man et al., 1999; Naeimi et al., 2010; Prasad and Kumar, 2011; Balasubramanian et al., 2014) and T. viride (Mathivanan et al., 2005; Pal et al., 2015) were successful in controlling sheath blight of rice (R. solani).T. harzianum with P. fluorescens helped in reducing nematode population by producing some special structure which contained toxins and alkaloids that killed eggs, juvenile stage and also inhibited growth and activity of nematodes (Narasimhamurthy et al., 2017). Jambhulkar et al. (2018) showed that the combined application of T. harzianum with P. fluorescens increased efficacy and consistency of the two biocontrol agents to impart a synergistic response against rice blast disease.
The formulations must maintain the antagonists in a viable form in the environment as long as it is necessary for the mode of action to be expressed and favours survival of biocontrol agents in the environment (Joshi and Raut, 2005). Although Trichoderma isolates of T8 and T21 exhibited moderate degree of antagonistic activity in dual culture experiments, these isolates provided higher antagonistic values against M. grisea that indicates an effective mycoparasitism against infective agent in soil (Bell et al., 1982; Harman, 2000; Schuster and Schmoll, 2010; Alfredo and Aleli, 2011). The antagonism of Trichoderma species is widely explored and this fungus provides advantages to guard roots from disease and pathogen attack. Trichoderma has ability to supply noxious substrate in growth media. The Trichoderma have antagonism and saprobe traits to plant pathogens (Suparno et al., 2016). The antagonistic property of Trichoderma sp. and P. fluorescens was determined against rice sheath blight (R. solani). On co-culturing, growth of the antagonists was noticed in individual media whereas growth of R. solani was suppressed (Anitha and Das, 2011).
Dennis and Webster (1971) observed in a study that T. viride significantly decreased sheath blight infection and increased grain yield in rice. Trichoderma inoculants into sheath blight plagued rice straw also resulted in decline of the extent of infestation caused by Rhizoctonia solani (Nagamani and Mew, 1987; Man and Noda, 1997). In another study, Baby and Manibhushanrao (1993) reported 25% increase in growth of rice plants after application of antagonists to soil. Patel and Mukhopadhyay (1997) found that spraying of fungal antagonists increased the grain weight in rice. The application of Trichoderma or different Trichoderma formulations like T. harzianum exhibited significant reduction of the disease as compared to various formulations (Table 2) and among bioagents, T. harzianum was found to be the best in managing sheath blight (R. solani) giving 48.2% and 30.0% reduction in disease severity and incidence, respectively (Khan and Sinha, 2007). Nevertheless, Sarkar et al. (2014) documented that the use of bioagents, including Trichoderma sp., for brown spot management was not so effective than the traditional application of fungicides although in vitro experiment showed some promise in controlling the pathogen.
Singh and Sinha (2009) demonstrated enhancement in the growth of rice crop after seed treatment with T. harzianum and Pseudomonas fluorescens (Table 4) which might be attributed to the enhanced nitrogen uptake and drought tolerance as a result of the bio control treatments. Trichoderma species facilitated production of growth hormones like auxins and gibberelins which supported root growth and increased water absorption capacity from soil (Contreras-Cornejo et al., 2009; Martínez-Medina et al., 2011).
Effect of Trichoderma on stress tolerance activity
T. harzianum strain is known for enhancing the yield and yield attributes of stress tolerant rice varieties (Zaidi et al., 2018) (Table 2). Application of T. harzianum enhanced salt tolerance of maize (Zea mays L.) and rice through higher inhibitor activities and high amino alkanoic acid content. This is not only due to the physiological parameters but also due to the reduction in H2O2 concentration, hence minimizing the damage created by reactive oxygen species (ROS) inside plants (Yasmeen and Zamin, 2017).
Shukla et al. (2012) unconcealed an immensely vital result of treatment with rice root dip in drought tolerant isolates of T. harzianum to alleviate drought stress. Trichoderma treatment relieved the strain condition, considerably improved length and contemporary weight of shoot and root, increased leaves range, leaf area, photosynthetic pigment content, and reduces stress levels (Rawat et al., 2012).
On the other hand, Pandey et al. (2016) found that T. harzianum down regulated the expression of Aquaporin, LOC_Os06g12310 gene expression of PSD-17 rice genotype under drought condition and its interaction improved drought.
Potent phosphate solubilizing activity that trends to increase soil fertility
Kapri and Tewari (2010) have documented the ability of Trichoderma to solubilized phosphate by releasing phosphatase enzymes, thereby enhancing soil fertility and plant growth.
In other study, Chagas et al. (2015) also found that the plant growth was considerably hyperbolic in presence of natural phosphate stirred by Trichoderma immunization when compared to plant growth management in absence of Trichoderma. Application of composted rice straw with Trichoderma spp. helped to maintain soil pH between 4.2 and 4.51, which was not toxic to rice plant growth and the nutrient content in the rice soil. Organic carbon, available N, P and exchangeable K was also improved considerably as compared to the conventional application by the farmers dose burnt rice straw + 100 % (100N-60P2O5-30 K2O kg ha-1) reported by Son et al. (2013).
Improvement of seedling vigor and promotion of seed germination
Trichoderma species that has the ability to promote rice seedling length can be an alternative way to be used in sustainable crop production. The length of shoot and root of the seedling were significantly increased by using Trichoderma species in comparison to control (in absence of Trichoderma spp.). Auxins produced with the help of Trichoderma spp. are able to stimulate root initiation and cell elongation while gibberellins are involved in cell division. Stimulation of production of auxins and gibberellins by Trichoderma spp. enhances rice seedling performance during seed germination (Doni et al., 2014b).
Role in decomposition, myco-parasitism and cellulose degradation
Trichoderma spp. are beneficial fungi that are present in substantial numbers in nearly all agricultural soils and in different environments like decaying wood (Harman et al., 1993). It grows typically towards hyphae of different fungi, coil them in a lectin-mediated reaction and degrade the target fungi cell walls by a method called mycoparasitism (Harman et al., 1993).
Microscopic observation suggests that T. harzianum produces and excretes mycolytic enzymes that lyse cell membrane during interaction (Carsolio et al., 1994). The extracellular secretion of 1,3-ß glucanases, chitinases and proteinase facilitated by T. harzianum play a vital role in biocontrol (Carsolio et al., 1994). The antagonistic process starts with physical contact and T. viride is an antagonist of soil-borne fungal pathogens. The enzyme weakens the host cell wall and increases the rate of diffusion of the antibiotics through the cell wall. Upon physical contact the T. viride coils around its host where it proceeds with invasive growth. The host hyphae collapse because of loss of turgor pressure (Harman et al., 1993).
Increased absorption of nutrients and soil fertility
T. asperellum is known to increase the ability of plants to absorb the metallic component that may be responsible for higher chemical processes (Harman et al., 2004; De Santiago et al., 2010). Trichoderma considerably increased the macronutrient uptake and water acquisition capabilities through promotion of stronger root systems (Doni et al., 2014a). The flexibility of Trichoderma to modulate rice plants N, P and K uptake was presumptively because of its capability to supply cellulolytic enzymes, which degrade polysaccharide in soils and releases organic N, P and K within the rice rhizosphere. Jiang et al. (2011) reported this ability of Trichoderma.
Trichoderma possesses mechanisms to promote plant growth through discharge of indole-3-acetic acid that in turn increases root growth and thereby plays a significant role in nutrient acquisition (Hoyos-Carvajal et al., 2009). The presence of Trichoderma in the soil results in the release of mineral elements such as macronutrients (K, P, Ca) and micronutrient (Zn) in the soil which is vigorously absorbed by the plants. Such type of nutrition helps in high dry matter production during vegetative stage and promotes early flowering and fruiting resulting in higher yields (Cuevas, 2006). Trichoderma treated plants were able to enhance nutrient uptake, resulting in increased root and shoot growth and improved plant vigour ensuring rapidly growth with enhanced plant greenness due to higher photosynthetic rates, hence increased carbohydrate as well as biomass production (Harman, 2006; Saba et al., 2012). Trichoderma species increased the biological nitrogen fixation in soil and nitrogen uptake capacity of the plant (Dordas and Sioulas, 2008).
Growth inhibition of deleterious root microflora
The information documented by Kumar et al. (2017b) showed the effective implementation of Trichoderma along with agrochemicals for suppressing root knot nematode development and soil born fungal pathogens during rice cultivation (Table 1). This nematophagus fungus developed some special structures that killed the eggs and juvenile by producing toxins and alkaloids, which hindered the growth and activity of nematodes (Narasimhamurthy et al., 2017).
Enhanced plant development and growth promotion
Several scientific reports revealed that Trichoderma isolates induce growth of plants in various environments (Bell et al., 1982; Harman, 2000; Schuster and Schmoll, 2010; Alfredo and Aleli, 2011; Ali and Nadarajah, 2014; Nongmaithem et al., 2017). Therefore, Trichoderma species have a positive impact in promoting plant growth and development in rice. This ability could be attributed to several mechanisms (Figure 1). Some of them are mycoparasitism, antibiosis, degradation of toxins, inactivation of pathogenic enzymatic pathways, resistance to pathogens, enhanced nutrient uptake, solubilization, sequestration of inorganic nutrients and enhancement of root hair development (Harman, 2006; Lorito et al., 2010). In this sense, Shukla et al. (2012) informed that T. harzianum significantly increased the ability of rice plants to tolerate drought stress and increase rice water holding capacity, induced higher leaf number and tiller number. Besides, Kumar et al. (2017a) reported that T. viride extensively improved the plant growth with respect to height of the plant. Application of Trichoderma species with farmer practice revealed a significant increase rice growth components (Doni et al., 2014c; Adak et al., 2016) and higher number of effective tillers over the control (Zaidi et al., 2018). Kurrey et al. (2018) also demonstrate that hydrogel and Trichoderma enhance the root development and water productivity and those are effective in improving soil moisture availability and thus increased plant establishment.
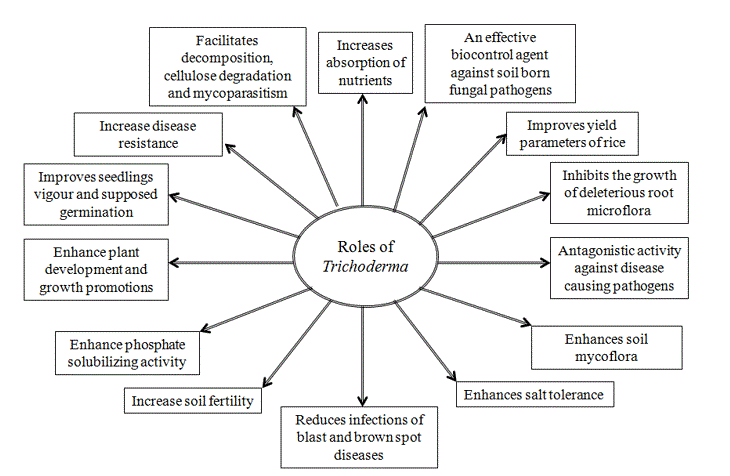
Figure 1. Impact of Trichoderma species on soil and plants health during rice cultivation by positively effecting rice plant growth and yield.
CONCLUSIONS
Present review gives an overall involvement of different Trichoderma species in improving growth parameters, increasing grain yield, improving soil fertility, decreasing chemical fertilizer use hence reducing the cost involved for fertilization and enhancing economic effectiveness. Present finding suggests that use of Trichoderma singly or in combination with other microorganism (biocontrol agents) may be a valuable tool in managing different diseases of rice and it has an effective use in natural abiotic stress tolerance and is one of the best management practices of crop to alleviate stresses occurring in field conditions. Because Trichoderma treatments appreciably alleviates the growth parameter effects of hyper salinity, seed biopriming treatment may be used for getting better plant growth and yield of rice in saline condition. There may be some active metabolites or compounds produced by Trichoderma species which stimulate different biological activities of plant. The overall findings reveal Trichoderma species to be an effective biocontrol agent that needs to be commercialized throughout the world several formulations are commercialize around the World. The farmers can use Trichoderma species as a biofertilizer or biocontrol because it has no negative effect either on the soil and the environment. Due to the activities rendered by Trichoderma species, it is expected that in near future, exploitation of this fascinating microorganism would be maximized under organic agriculture in rice.
ACKNOWLEDGMENTS
The first author is thankful to the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India for the financial assistance received through Multi-Institutional project (Sanctioned Order no. DBT-NER/AGRI/29/2015). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Author contributions
Conceptualization, Investigation and Writing-original draft SD, Resources GC, Writing-review & editing SSD, Methodology and Formal analysis SRC, Supervision PD and AKS.
REFERENCES
Adak A, Prasanna R, Babu S, Bidyarani N, Verma S, Pal M, Shivay YS, Nain L (2016) Micronutrient enrichment mediated by plant microbe interactions and rice cultivation practices. J Plant Nutr 39(9): 1216-1232; doi:10.1080/01904167.2016.1148723
Adesemoye AO, Kloepper JW (2009) Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biot 85: 1-12; doi:10.1007/s00253-009-2196-0
Alfredo MS, Aleli RP (2011) Biological control of sheath blight of upland rice with Trichoderma species. J Trop Plant Pathol 69: 1-9
Ali H, Nadarajah K (2014) Evaluating the efficacy of Trichoderma spp and Bacillus subtilis as biocontrol agents against Magnaporthe grisea in rice. AJCS 8(9): 1324-1335
Anitha A, Das MA (2011) Activation of rice plant growth against Rhizoctonia solani using Pseudomonas fluorescens, Trichoderma and salicylic acid. Research in Biotechnology 2(4): 07-12
Baby UI, Manibhushanrao K (1993) Control of rice sheath blight disease by organic manures. In: Ravikumar R (ed). Proceedings of the fifth Kerala Science Congress, Organized by State Commission on Science, Technology and Environment, Government of Kerala, pp. 158-161. Thiruvananthapuram, India
Balasubramanian N, Priya VT, Shanmugaiah V, Lalithakum D (2014) Effect of improved Trichoderma fusants and their parent strains in control of sheath blight of rice and wilt of tomato. J Plant Dis Protect 121(2): 71-78; doi:10.1007/BF03356494
Banayo NPM, Cruz PCS, Aguilar EA, Badayos RB, Haefele SM (2012) Evaluation of biofertilizers in irrigated rice: effects on grain yield at different fertilizer rates. Agriculture 2: 73-86; doi:10.3390/agriculture2010073
Bishwajit G, Sarker S, Kpoghomou MA, Gao H, Jun L, Yin D, Ghosh S (2013) Self sufficiency in rice and food security: a South Asian perspective. Agric Food Secur 2: 10; doi:10.1186/2048-7010-2-10
Carsolio C, Gutiérrez A, Jiménez B, Van Montagu M, Herrera-Estrella A (1994) Characterization of ech-42, a Trichoderma harzianum endochitinase gene expressed during mycoparasitism. Proc Natl Acad Sci USA 91(23): 10903-10907; doi:10.1073/pnas.91.23.10903
Chagas LFB, Chagas Junior AF, Carvalho MR, Miller LO, Colonia BSO (2015) Evaluation of the phosphate solubilization potential of Trichoderma strains (Trichoplus JCO) and effects on rice biomass. J Soil Sci Plant Nutr 15(3): 794-804; doi:10.4067/S0718-95162015005000054
Chen Q, Tao S, Bi X, Xu X, Wang L, Li X (2013) Research progress in physiological and molecular biology mechanism of drought resistance in rice. Am J Mol Biol 3: 102-107; doi:10.4236/ajmb.2013.32014
Crosson P (1995) Natural resource and environmental consequences of rice production Los Banos, Manila, Philippines. In: International Rice Research Institute (Ed). Fragile Lives in Fragile Ecosystems, pp. 83-100. International Rice Research Conference, Manila
Cuevas VC (2006) Soil inoculation with Trichoderma pseudokoningii Rifai enhances yield of rice. Philipp J Sci 135(1): 31-37
Cuevas VC, Sinohin AM, Arro EA JR (2001) Efficacy of Trichoderma spp. as biological control agent of Sclerotium rolfsii Sacc. The Philipp Agricul Scient 84(1): 35-42
Cuevas VC, Sinohin AM, Orajay JI (2005) Performance of selected Philippine species of Trichoderma as biocontrol agent of damping-off pathogens and as growth enhancer of vegetables in farmers’ field. The Philipp Agricul Scient 88(1): 37-45
Cuevas VC, Soriano JM, Bagunu LG, Soniega JA, Alfonso AL (1995) Control of damping-off diseases of vegetables by Trichoderma species. Phil Agr 78: 255-276
Dar MH, de Janvry A, Emerick K, Raitzer D, Sadoulet E (2013) Flood-tolerant rice reduces yield variability and raises expected yield, differentially benefitting socially disadvantaged groups. Sci Rep 3: 3315; doi:10.1038/srep03315
De Santiago A, Quintero JM, Avilés M, Delgado A (2010) Effect of Trichoderma asperellum strain T34 on iron, copper, manganese, and zinc uptake by wheat grown on a calcareous medium. Plant and Soil 342(1-2): 97-104; doi:10.1007/s11104-010-0670-1
Dennis C, Webster J (1971) Antagonistic properties of species-groups of Trichoderma. Trans Br Mycol Soc 57(1): 41-IN4; doi:10.1016/s0007-1536(71)80078-5
Doni F, Zain CRCM, Isahak A, Fathurrahman F, Sulaiman N, Uphoff N, Yusoff WMW (2017) Relationships observed between Trichoderma inoculation and characteristics of rice grown under System of Rice Intensification (SRI) vs. conventional methods of cultivation. Symbiosis 72: 45-59; doi:10.1007/s13199-016-0438-3
Doni F, Anizan I, Che Radziah CMZ, Salman AH, Rodzihan MH, Yusoff WMW (2014b) Enhancement of rice seed germination and vigour by Trichoderma spp. Res J Appl Sci Eng Tech 7(21): 4547-4552; doi:10.19026/rjaset.7.832
Doni F, Anizanb I, Che Radziah CMZ, Yusoffa WMW (2014d) Transpiration rates of rice plants treated with Trichoderma spp. AIP Conf Proc 1614: 566-569; doi:10.1063/1.4895263
Doni F, Isahak A, Zain CRCM, Ariffin SM, Mohamad WNW, Yusoff WMW (2014a) Formulation of Trichoderma sp. SL2 inoculants using different carriers for soil treatment in rice seedling growth. Springerplus 3: 532; doi:10.1186/2193-1801-3-532
Doni F, Isahak A, Zain CRM, Yusoff WMW (2014c) Physiological and growth response of rice plants (Oryza sativa L.) to Trichoderma spp. inoculants. AMB Express 4: 45; doi:10.1186/s13568-014-0045-8
Dordas C, Sioulas C (2008) Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind Crops Prod 27: 75-85; doi:10.1016/j.indcrop.2007.07.020
Druzhinina IS, Kopchinsky AG, Kubicek CP (2006) The first 100 Trichoderma species characterized by molecular data. Mycoscience 47: 55-64; doi:10.1007/S10267-006-0279-7
Felix CR, Noronha EF, Miller RNG (2014) Trichoderma: A Dual Function Fungi and Their Use in the Wine and Beer Industries. In: Gupta VK, Schmoll M, Herrera-Estrella A, Upadhyay RS, Druzhinina I, Tuohy MG (eds). Biotechnology and Biology of Trichoderma, pp. 345-349. Elsevier, Oxford; ISBN: 9780444595768
França SKSD, Cardoso AF, Lustosa DC, Ramos EMLS, Filippi MCCD (2015) Biocontrol of sheath blight by Trichoderma asperellum in tropical lowland rice. Agron Sustain Dev 35(1): 317-324; doi:10.1007/s13593-014-0244-3
Gomathinayagam S, Persaud SA, Rekha M (2012) Comparative study of biological agents, Trichoderma harzianum and Trichoderma viride for controlling brown spot disease in rice. JBiopest 5: 28-32
Gomathinayagam S, Rekha M, Murugan SS, Jagessar RC (2009) Biological control of rice disease (blast) by using Trichoderma viride in laboratory conditions. Proceedings of the Caribbean Food Crops Society 45: 138-140; doi:10.22004/ag.econ.256340
Harman GE (2000) Myths and Dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzinum T-22. Plant Dis 84(4): 377-393
Harman GE (2006) Overview of mechanisms and uses of Trichoderma spp. Phytopathol 96: 190-194
Harman GE, Hayes CK, Lorito M, Broadway RM, Di Pietro A, Tronsmo A (1993) Chitinolytic enzymes of Trichoderma harzianum, purification of chitibiosidase and endochitinase. Phytopathology 83: 313-318
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2: 43-56; doi:10.1038/nrmicro797
Hermosa R, Viterbo A, Chet I, Monte E (2012) Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158: 17-25; doi:10.1099/mic.0.052274-0
Hoyos-Carvajal L, Orduz S, Bissett J (2009) Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol Control 51: 409-416; doi:10.1016/j.biocontrol.2009.07.018
Huang JZ, Guo EZ, Zhang HL, Shu QY (2014) Workable male sterility systems for hybrid rice: genetics, biochemistry, molecular biology and utilization. Rice 7: 1-14; doi:10.1186/s12284-014-0013-6
Jambhulkar PP, Sharma P, Manokaran R, Lakshman DK, Rokadia P, Jambhulkar N (2018) Assessing synergism of combined applications of Trichoderma harzianum and Pseudomonas fluorescens to control blast and bacterial leaf blight of rice. Eur J Plant Pathol 152: 747-757; doi:10.1007/s10658-018-1519-3
Ji K, Wang Y, Sun W, Lou Q, Mei H, Shen S, Chen H (2012) Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J Plant Physiol 169: 336-344; doi:10.1016/j.jplph.2011.10.010
Jiang X, Geng A, He N, Li Q (2011) New Isolate of Trichoderma viride strain for enhanced cellulolytic enzyme complex production. J Biosci Bioeng 111: 121-127; doi:10.1016/j.jbiosc.2010.09.004
Joshi V, Raut SP (2005) Promising bio-agents in management of Fusarium solani causing seedling wilt of cashew hybrid. Annals of Pl Prot Sci 13(1):0-0
Kannahi M, Dhivya S, Senthilkumar R (2016) Biological control on rice false smut disease using Trichoderma species. Int J Pure App Biosci 4(2): 311-316; doi:10.18782/2320-7051.2237
Kapri A, Tewari L (2010) Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz J Microbiol 41(3): 787-795; doi:10.1590/S1517-83822010005000031
Khadka RB, Uphoff N (2019) Effects of Trichoderma seedling treatment with System of Rice Intensification management and with conventional management of transplanted rice.PeerJ 7: e5877; doi:10.7717/peerj.5877
Khan AA, Singh R (2015) Influence of zinc on Trichoderma harzianum and sheath blightof rice under glasshouse conditions. Int J Plant Prot 8(2): 303-306; doi:10.15740/HAS/IJPP/8.2/303-306
Khan AA, Sinha AP (2007) Biocontrol potential of Trichoderma species against sheath blight of rice. Indian Phytopath 60(2): 208–213
Khan HI (2018) Appraisal of biofertilizers in rice: to supplement the inorganic chemical fertilizer. Rice Sci 25(6): 357-362; doi:10.1016/j.rsci.2018.10.006
Kumar D, Khilari K, Kumar N, Jain SK (2017a) Integrated Disease Management of rice root knot nematode (Meloidogyne graminicola) through organic amendments, Trichoderma spp. and Carbofuran. J Pharmacogn Phytochem 6(6): 2509-2515
Kumar H, Ahmad S, Zacharia S, Kumar D, Khan A (2016) Growth promotion of transplanted rice plant by fungal and bacterial bioagents effective against brown leaf spot caused by Drechslera oryzae. J Pure Appl Microbio 10(3): 2394-2395
Kumar S, Lal AA, Kumar N, Jaiswal S, Kumar H, Kumar A, Kumar M (2017b) Effect of bio control agents and botanicals against Blast of Paddy caused by Pyricularia oryzae. Int J Chem Stud 5(1): 314-318
Kurrey D, Singh RK, Rajput RS (2018) Effect of Hydrogel and Trichoderma on root growth and water productivity in rice varieties under Rainfed Conditions. Res J Agric Sci 9: 210-212
Lorito M, Woo SL, Harman GE, Monte E (2010) Translational research on Trichoderma: from ’omics to the field. Annu Rev Phytopathol 48: 395-417; doi:10.1146/annurev-phyto-073009-114314
Man LH, Ha NN, Kon PSTT, Hiraoka H (1999) Integrated nutrient management for a sustainable agriculture at Omon, Vietnam, Japan. Omonrice 7: 96-103
Man LH, Ha NN (2006) Effect of decomposed rice straw at different times on rice yield. Omonrice 14: 58-63
Man LH, Noda T(1997) Trichoderma fungus as biological agent to Rhizoctonia solani and rice straw decomposition, Results on Science Research in CLRRI (1977-1997). Agriculture Publisher, Ho Chi Minh
Martínez-Medina A, Roldán A, Albacete A, Pascual JA (2011) The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry 72: 223-229; doi:10.1016/j.phytochem.2010.11.008
Mastouri F, Bjorkman T, Harman GE (2010) Seed treatments with Trichoderma harzianum alleviates biotic, abiotic and physiological stresses in germinating seeds and seedlings. Phytopathology 100: 1213-1221; doi:10.1094/PHYTO-03-10-0091
Mathivanan, NN, Prabavathy,VR. Vijayanandraj VR (2005) Application of talc formulations of Pseudomonas fluorescens Migula and Trichoderma viride pers. ex S.F. gray decrease the sheath blight disease and enhance the plant growth and yield in rice. J Phytopathology 153: 697-701; doi:10.1111/j.1439-0434.2005.01042.x
Mukherjee PK, Horwitz BA, Herrera-Estrella A (2013) Trichoderma research in the genome era. Annu Rev Phytopathol 51: 105-29; doi:10.1146/annurev-phyto-082712-102353
Naeimi S, Okhovvat SM, Javan-Nikkhah M, Vágvölgyi C, Khosravi V, Kredics L (2010) Biological control of Rhizoctonia solani AG1-1A, the causal agent of rice sheath blight with Trichoderma strains. Phytopathol Mediterr 49: 287-300
Nagamani A, Mew TW (1987) Trichoderma in Philippine rice field soils. International Rice Research Newsletter 12(4): 25
Narasimhamurthy HB, Ravindra H, Sehgal M, Ekabote SD, Ganapathi G (2017) Bio-management of rice root-knot nematode (Meloidogyne graminicola). J Entomol Zool Stud 5(4): 1433-1439
Ng LC, Ngadin A, Azhari M, Zahari NA (2015) Potential of Trichoderma spp. as biological control agents against Bakanae Pathogen (Fusarium fujikuroi) in Rice. Asian J Plant Pathol 9(2): 46-58; doi:10.3923/ajppaj.2015.46.58
Nongmaithem N, Roy A, Bhattacharya PM (2017) Potential of Trichoderma spp. on growth promotion and mitigating cadmium uptake in rice plant under the metal stress ecosystem. Int J Curr Microbiol App Sci 6(6): 992-1010; doi:10.20546/ijcmas.2017.606.116
Pal R, Biswas MK, Mandal D, Naik BS (2015) Management of sheath blight disease of rice through bio control agents in west central table land zone of Odisha. Int J Adv Res 3(11): 747-753
Pandey V, Ansari MW, Tula S, Yadav S, Sahoo RK, Shukla N, Bains G, Badal S, Chandra S, Gaur AK, Kumar A, Shukla A, Kumar J, Tuteja N (2016) Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 243: 1251-1264; doi:10.1007/s00425-016-2482-x
Patel LA, Mukhopadhyay S (1997) Mass multiplication of antagonists and standardization of effective dose for management of sheath blight in rice. Eco and Envt Sci 3: 206-209
Person CH (1794) Dispositio methodica fungorum. Neues Magazin für die Botanik 1:81–128
Prasad BN, Kumar MR (2011) Comparative efficacy of different isolates of Trichoderma spp. against Rhizoctonia solani, incitant of sheath blight of rice. Indian Journal of Fundamental and Applied Life Sciences 1(3): 107-111
Rahman M, Ali J, Masood M (2015) Seed priming and Trichoderma application: a method for improving seedling establishment and yield of dry direct seeded Boro (Winter) rice in Bangladesh. Univers J Agric 3(2): 59-67; doi:10.13189/ujar.2015.030205
Rawat L, Singh Y, Shukla N, Kumar J (2012) Seed biopriming with salinity tolerant isolates of Trichoderma harzianum alleviates salt stress in rice: growth, physiological and biochemical characteristics. J Plant Pathol 94(2): 353-365; doi:10.4454/JPP.FA.2012.026
Saba H, Vibhash D, Manisha M, Prashant KS, Farhan H, Tauseef A (2012) Trichoderma – a promising plant growth stimulator and biocontrol agent. Mycosphere 3(4): 524-531; doi:10.5943 /mycosphere/3/4/14
Samuels GJ (1996) Trichoderma: a review of biological systemics of the genus. Mycol Res 100(8): 923-935
Sannathimmappa HG, Gurumurthy BR, Jayadeva HM, Rajanna D, Shivanna MB (2015) Effect of paddy straw based integrated nutrient management practices for sustainable production of rice. IOSR Journal of Agriculture and Veterinary Science 8(1): 74-77
Sarkar D, Mandal R, Roy P, Taradar J, Dasgupta B (2014) Management of brown spot disease of rice by using safer fungicides and some bioagents. The Bioscan 9(1): 437-441
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotech 87: 787-799; doi:10.1007/s00253-010-2632-1
Shoresh M, Harman GE (2008) The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: a proteomic approach. Plant Physiol 47(4): 2147-2163; doi:10.1104/pp.108.123810
Shukla N, Awasthi RP, Rawat L, Kumar J (2012) Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol Biochem 54: 78-88; doi:10.1016/j.plaphy.2012.02.001
Singh A, Sarma BK, Singh HB, Upadhyay RS (2014) Trichoderma. Biotechnology and Biology of Trichoderma 0 (0): 533-542
Singh R, Singh GS (2017) Traditional agriculture: a climate-smart approach for sustainable food production. Energ Ecol Environ 2: 296-316; doi:10.1007/s40974-017-0074-7
Singh R, Singh R, Singh US, Kumar A (2018) Population of Trichoderma harzianum strains in rice rhizospheres at different interval after seed sowing. Int J Chem Stud 6(2): 2829-2831
Singh R, Sinha AP (2009) Biological control of rice sheath blight with antagonistic bacteria. Ann PI Protec Sci 17: 107-110
Son TTN, Thu TTA, Nam NN, Man LH (2013) Influence of rice straw treated by indigenous Trichoderma spp. on soil fertility, rice grain yield and economic efficiency in the Mekong Delta. Omonrice 19: 145-152
Subhalakshmi T, Devi IS (2017) Blast of rice in Manipur and its biocontrol by Pseudomonas fluorescens and Trichoderma sp. Int J Curr Microbiol App Sci 6(6): 1619-1634; doi:10.20546/ijcmas.2017.606.190
Suparno S, Hakim L, Aidawati N (2016) Trichoderma spp. as agent of biological control in local rice diseases in tidal swamp lands in South Kalimantan, Indonesia. IOSR Journal of Agriculture and Veterinary Science 9(1): 01-06
Swain H, Adak T, Mukherjee AK, Mukherjee PK, Bhattacharyya P, Behera S, Bagchi TB, Patro R, Shasmitaa, Khandual A, Bag MK, Dangar TK, Lenka S, Jena M (2018) Novel Trichoderma strains isolated from tree barks as potential biocontrol agents and biofertilizers for direct seeded rice. Microbiol Res 214: 83-90; doi:10.1016/j.micres.2018.05.015
Taufik M, Wijayanto T, Gusnawaty HS, Nurmas A, Alam S, Santiaji LO, Sarawa (2016) Improvement of local upland rice utilizing mixture of microbes: resistance, yield and reduction of chemical fertilizer usage. Int J Biosci 9(5): 97-107; doi:10.12692/ijb/9.5.97-107
Vejan P, Abdullah R, Khadiran T, Ismail S, Boyce AN (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability - a review. Molecules 21: 573-590; doi:10.3390/molecules21050573
Verma M, Brar SK, Tyagi RD, Sahai V, Prévost D, Valéro JR, Surampalli RY (2007) Bench-scale fermentation of Trichoderma viride on wastewater sludge: rheology, lytic enzymes and biocontrol activity. Enzyme Microb Technol 41: 764-771; doi:10.1016/j.enzmictec.2007.06.013
Woo SL, Ruocco M, Vinale F, Nigro M, Marra R, Lombardi N (2014) Trichoderma-based products and their widespread use in agriculture. Open Mycol J 8:71-126; doi:10.2174/1874437001408010071
Xu XM, Jeffries P, Pautasso M, Jeger MJ (2011) Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 101: 1024-1031; doi:10.1094/PHYTO-08-10-0216
Yasmeen R, Zamin SS (2017) Physiological responses of crop plants against Trichoderma harzianum in saline environment. Acta Bot Croat 76(2): 154-162; doi:10.1515/botcro-2016-0054
Zaidi MW, Singh M, Kumar S, Sangle UR, Nityanand SR, Sachitanand Prasad, Rameshwar, Singh SS, Singh S, Yadav AK, Singh A, Showkat A, Singh US (2018) Trichoderma harzianum improves the performance of stress tolerant rice varieties in rain fed ecologies of Bihar, India. Field Crop Res 220: 97-104; doi:10.1016/j.fcr.2017.05.003
Supplementary material
Table S1. Application of Trichoderma spp. as plant growth promoter in rice cultivation. (TableS1.pdf)
Received: 08-02-2020
Accepted: 17-02-2020
Copyright (c) 2020 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
