Artículo original
Biotecnología Vegetal Vol. 20 No. 2: 92 - 103 , abril - junio, 2020
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
In vitro propagation of Peperomia albovittata and Peperomia galioides by organogenesis
Propagación in vitro de Peperomia albovittata y Peperomia galioides por organogénesis
Consuelo Rojas-Idrogo1,2 https://orcid.org/0000-0001-5769-8209
Guillermo E. Delgado-Paredes1,2 https://orcid.org/0000-0003-3525-6711
1Laboratorio de Cultivo de Tejidos Vegetales y Recursos Genéticos, Facultad de Ciencias Biológicas, Universidad Nacional Pedro Ruiz Gallo. Ciudad Universitaria, Juan XXIII No 391. Lambayeque. Perú.
2Laboratorio General de Biotecnología, Vicerrectorado de Investigación (UNPRG), Universidad Nacional Pedro Ruiz Gallo. Atahualpa No 423. Lambayeque. Perú.
*Corresponding author e-mail: guidelg2015@yahoo.es
ABSTRACT
Peperomia albovittata and P. galioides are two herbaceous species, belonging to the Piperaceae family, collected in the Andean district of Kañaris (1128 to 1550 m above sea level), in Lambayeque region, Peru. These species are recalcitrant to propagation by conventional vegetative methods, whereby the present study aims to in vitro propagate of Peperomia albovittata and P. galioides through by organogenesis. Single nodes with axillar buds, stem segments (internodes), leaves and petioles were cultured on gellified basal culture medium and was added IAA-GA3, NAA-GA3 and NAA-BAP at different concentrations. Only in the NAA-GA3 combination clonal propagation from nodal segments and direct organogenesis in internodes were observed. Callus induction and proliferation was observed in some interactions of NAA-BAP, although all explants from leaves turned dark brown and died. On the other hand, shoot regeneration was observed only in P. galioides. Both species, especially P. albovittata, showed a high degree of recalcitrance to the induction of in vitro morphogenic processes.
Keywords: Callus induction, clonal propagation, indirect organogenesis, NAA-GA3 combination, vitrification and phenolic browning
RESUMEN
Peperomia albovittata and P. galioides son dos especies herbáceas, pertenecientes a la familia Piperaceae, colectadas en el distrito andino de Kañaris (1128 a 1150 msnm, en la región Lambayeque, Perú. Estas especies son recalcitrantes a la propagación por métodos vegetativos convencionales, por lo que el objetivo de este trabajo de investigación fue propagar Peperomia albovittata y P. galioides, mediante organogénesis. Nudos con yemas axilares, segmentos de tallos (entrenudos), hojas y peciolos fueron cultivados en medio de cultivo semisólido y complementado con diferentes concentraciones de AIA-AG3, ANA-AG3 y ANA-AG3 y ANA-BAP. Solamente en la combinación ANA-AG3, fue observada la propagación clonal, a partir de segmentos nodales, y la organogénesis directa en entrenudos. La inducción y proliferación de callos fue observada en algunas interacciones de ANA-BAP, aunque todos los explantes de hojas tomaban un color marrón oscuro y morían. Por otro lado, la regeneración de brotes fue observada solamente en P. galioides. Ambas especies, especialmente P. albovittata, mostraron un alto grado de recalcitrancia a la inducción de procesos morfogénicos in vitro.
Palabras clave: Combinación ANA-AG3, inducción de callos, organogénesis indirecta, propagación clonal, vitrificación y oxidaci�n fenólica
INTRODUCTION
The family Piperaceae, of the perianthless members of the Piperales, together with the primitive families Chlorantaceae and Saururaceae have been considered as basal and ancestral Angiospermae due to its very reduced flowers (Jaramillo et al., 2004). The genera Peperomia and Piper are the largest ones, including more than 2000 species (Parmar et al., 1997; Mathieu et al., 2015). Peperomia R. & P. (Piperaceae) is a genus with a pantropical distribution that in America occurs from Bermuda to Argentina and Uruguay (Boufford, 1982; Wanke et al., 2006; Mathieu et al., 2015; IBODA, 2015; Mai et al., 2016). The genus Peperomia includes 1432 herbaceous, terrestrial or epiphytic species (Guimarães et al., 1984; Mathieu et al., 2015). In Peru, the Peperomia genus includes around 381 species (Brako and Zarucchi, 1993), with approximately 226 endemic species, most of which are highly valued as ornamental plants.
In spite of the extensive literature about the Peperomia genus (Yuncker, 1953; Yuncker, 1974; Mai et al., 2016), the taxonomy of this genus is still very complex. For example, it has recently been reported that twelve of the estimated 1500-1700 taxa in Peperomia show a “pseudo-epiphyllous” inflorescences, a terminal inflorescences seem to originate from the base of a sessile leaf.
In Peru, two species: P. albovittata and P. galioides with this feature are known from herbarium collections (Mathieu et al., 2008). Likewise, the historical and traditional classification of Dahlstedt (1900), has some incongruencies (Samain et al., 2007), being necessary a new infrageneric classification with morphological and molecular data (Jaramillo et al., 2004; Wanke et al, 2006; Frenzke et al., 2015). In this regard, P. galioides belongs to the subgenus Micropiper, whileP. albovittata part to the subgenus Multipalmata (Frenzke et al., 2015).
Several Peperomia species have been used in the medicine showing different biological activities as antiparasitic, antimicrobial, antiviral and antitumoral (Gutierrez et al., 2016). In this sense, methanol extract of stems and leaves from P. pellucida showed a significant analgesic activity in mice (Aziba et al., 2001). In this same species, oral administration of the aqueous extract from the aerial parts was tested for anti-inflammatory and analgesic activities in rats and mice, respectively (de Fátima et al., 2004). The anti-fungal activities of seven chromenes isolated from P. villipetiola were evaluated against Cladosporium cladosporioides and C. sphaerospermum and two compounds, 5-methanol-7-methoxy-2,2-dimethyl-2H-1-chromene-6-carboxylic acid and methyl 5-acetoxymethanol-7-hydroxy-2,2-dimethyl-2H-1-chromene-6-carboxylate, were found to be the most active (Malquichagua et al., 2005). Likewise, from the aerial parts of P. blanda were isolated two well-known flavones and five tetrahydrofuran lignans showing high in vitro activity against epimastigotes form of Trypanosoma cruzi (Felippe et al., 2008). Antifungal activity of essential oils from leaves of several Piper species and Peperomia obtusifolia against several species of Candida and Cryptococcus neoformans was determined. However, P. obtusifolia did not show any biological activity (Morandim-Giannetti et al., 2010). In vitro cytotoxic properties of aqueous and ethanolic extracts of P. pellucida against HT-29 (Human, Colon carcinoma) cell lines were evaluated, and this cytotoxic potential increases when the plant extract also increases (Narayana et al., 2018).
Phytochemical studies of Peperomia species have evidenced the accumulation of several secondary metabolites. Leaves and stems extracts of P. villipetiola has been found to contain myristicin and seven chromenes, suggesting that orsellinic acid may be a common intermediate in their biosynthesis (Malquichagua et al., 2005). The phytochemical profile of essential oil from in vitro grown P. obtusifolia showed 16 volatile compounds and the major components identified were caryophyllene and apiol (Ilyas et al., 2014). Bioactive compounds of P. pellucida extracted with ethanol were identified where apiol was found to be a major component (Narayana et al., 2015). Chemical profiling and biological activity of P. blanda, used as a traditional remedy for serious diseases such as cancer by Yemeni people were determined, evaluating the effect of peperomin A and N,N´-diphenethyloxamide against several cancer cell lines (Al-Madhagi et al., 2018). In a recent scientific literature review, covering approximately 30 years of research, the occurrence, biogenesis and activity of natural products from Peperomia were studied, where typical classes of secondary metabolites were characterized as polyketides, chromenes, lignans and amides, resulting some of these very specific to Peperomia (Gutierrez et al., 2016).
In addition, P. metallica contain the palisade parenchyma, which mainly consists of a single cell layer with giant chloroplasts (2-6 per palisade cell), used as model object for electrophysiological studies (Bartels, 1965; Bulychev et al., 1972). Likewise, Peperomia pellucida, a cosmopolitan species with a rich history of medicinal uses, has gained attention since the several concentrations of naturally ocurring radionuclides as 238U, 230Th, 238 Ra, 210Pb, and others was determined in aerial parts (leaves and stems) and roots, and in the surrounding soil (Sussa et al., 2013). On the other hand, plant tissue culture and expression of Escherichia coli heat-labile enterotoxin B subunit (LTB), were studied, and the synthetic LTB gene was introduced into P. pellucida by biolistic transformation method (Loc et al., 2010).
Several studies have shown that Peperomia species respond positively to in vitro clonal propagation by organogenesis using several explants such as shoot apex, nodal and stem segments and leaves. In MS culture medium (Murashige and Skoog, 1962) supplemented with different combinations of plant growth regulators indole-3-acetic acid (IAA), 6-benzylaminopurine (BAP), Zeatin and gibberellic acid (GA3), a highly efficient micropropagation protocol was developed for both P. metallica and P. peduncularis, and regeneration by direct organogenesis from leaf discs was observed (Ahmadabad and Bock, 2010). Both stem nodes and leaf segments of P. obtusifolia, native to Florida, Mexico and the Caribbean, were cultured on MS basal medium complemented with N6-furfuryladenine (KIN) and 1-Naphthaleneacetic acid (NAA). However, the leaf segments explants turned dark brown and died, and no shoots or callus were formed, while shoot regeneration from nodal segments increased by increasing KIN concentration (El-Naggar and Osman, 2014). In this same species, apical meristems and nodal segments were used for mass propagation at different concentrations of several cytokinins as BAP, thidiazuron (TDZ) and KIN in MS culture medium (Ilyas et al., 2014). Direct organogenesis from nodal meristems of P. pellucida was induced on MS medium with 2.0 mg l-1 BAP (Shekhawat and Manokari, 2015).
Considering the importance of Peperomia albovittata and P. galioides as ornamental plant and possible source of different metabolites, the present study aims to in vitro propagate of Peperomia albovittata and P. galioides through by organogenesis. In this regard, there are no prior studies related to plant tissue culture, biochemical and genetic aspects of these species.
MATERIALS AND METHODS
Plant material
Peperomia albovittata (Figure 1 a) and P. galioides (Figure 1 b) were collected from montane forest (1128 to 1550 m above sea level), in the Andean district of Kañaris, Lambayeque (Perú) and identified by Prof. Consuelo Rojas Idrogo, Department of Botany, Faculty of Biological Sciences (Universidad Nacional Pedro Ruiz Gallo, Lambayeque, Perú). The voucher specimens were deposited in the Herbarium Truxillense (HUT) of Universidad Nacional de Trujillo (La Libertad, Peru). The mother plants for in vitro studies were grown in greenhouse.

Figure 1. a. Plants of Peperomia albovittata C. DC. and b. Peperomia galioides Kunth.
Surface disinfection of explants and in vitro establishment
The explants (leaves, leaves with petiole, petioles, nodes and internodes) were washed with distilled water. It were surface desinfected with 70% (v/v) ethanol for 30 sec, washed with distilled water, dipped in 5% (v/v) Clorox® bleach for 10 min and washed three times with sterile distilled water, in a laminar airflow hood. The explants were cut, with 5.0 mm length, by sterile scissors and scalpel blades and were inoculated horizontally on the culture medium.
The nodes were used only in P. galioides and contained an axillary bud. In the process of callus induction and direct organogenesis, only leaves and petioles were used as explants for P. albovittata, while in P. galioides, leaves with petiole, nodes and internodes were also used.
Culture media and in vitro environment conditions
MS (Murashige and Skoog, 1962) nutrient culture medium was used for the experiments for shoots regeneration and roots induction, and also for callus induction and shoot regeneration (indirect organogenesis).
All media were supplemented with vitamins (100 mg l-1 myo-inositol and 1.0 mg l-1 thiamine HCl), 2.0% (w/v) sucrose and several concentrations and combinations of plant growth regulators.
The following treatments were tested on shoot regeneration and root induction: IAA-GA3 and NAA-GA3, and for callus induction and indirect organogenesis: NAA-BAP, 2,4-D, NAA, IAA and NAA-BAP-GA3. The control treatment was not supplemented with plant growth regulators. The pH of culture medium was adjusted to 5.8±0.1, and supplemented with 0.6% (w/v) agar-agar. The culture medium was dispensed into 18x150 mm culture tubes and sterilized by autoclave at 121 °C for 15 min.
Each treatment comprised 10 explants and was performed twice. The experiments were evaluated after 60 days (shoot regeneration) or 90-150 days (callus induction and shoot regeneration). The explants were placed under cool white fluorescent light at an intensity of 40-50 μmol m-2 sec-2 for a 16-h photoperiod at 20-24 °C .
Callus were not subcultured since they showed phenolic browning and eventually died. Plantlets evaluation considered the number of regenerated shoots, root formation and callus growth; likewise, some physiological characteristics such as phenolic browning and vitrification.
The following empirical scale was developed in the callus evaluation: -, without callus formation; +, callus covers 1/3 of the explants; ++, callus covers 1/2 to 2/3 of the explants; +++, callus covers the whole explant.
Hardening
In vitro regenerated plantlets were transplanted into 10 cm pots containing a mixture of vermiculite and peat moss (2:1). Irrigation was done whenever needed. The environmental conditions of the greenhouse were as follows: irradiance 50-55 kW h/m2, photoperiod 12/12 h (day/night) at 20-22 °C.
Statistical analysis
The statistical comparison was performed by one-way ANOVA using SPSS software, and means were compared using the Duncan test (p<0.05). Each of the trials was analyzed separately and the conditions of the experiment were kept constant. An unifactorial design (combination of growth regulators in each type of explant), the Kolmogorov-Smirnov normality test (p>0.05) and the Levene homogeneity test (p = 0.45) were used.
RESULTS
One of the biggest problems observed at the establishment stage of the in vitro culture was the microbial contamination of the explants, especially in P. albovittata. This contamination was caused by fungi and bacteria (Figure 2). When the explants were leaves, leaves with petioles, internodes and callus, the fungi, belonging to the genus Fusarium, completely cover the explant causing their death (Figure 2 b). This does not occur when the explant of P. galioi was a node and the axillary bud grows rapidly. In this case, the contaminant belonging to the genus Aspergillus, does not compromise the survival of the plantlet (Figure 3 b, d).

Figure 2. Peperomia albovittata in vitro propagation. a. and b. Explants contaminated by bacteria and fungi, c. Vitrification of regenerated buds d. Direct organogenesis, e. In vitro plantlets at the moment of the soil transfer, f. Plants transferred to soil and acclimated in greenhouse conditions.

Figure 3. Peperomia galioides in vitro propagation. a and b. Propagation of nodes, c. Plants regeneration on callus from leaves tissue, d. Regenerated plant.
The second problem in the establishing of in vitro cultures was vitrification (Figure 2 c) and phenolic browning of the explants.
Likewise, the release of phenolic compounds by all explants, was started 48 hours after the culture was installed and as time passed the tissues were necrotic and died. Phenolic browning was observed mainly in treatments supplemented with cytokinins, and although successive transfer to fresh culture media is one of the recommendations to overcome this physiological problem. However, in this case it did not work because the necrosis in advanced stages resulted in the death of the tissues.
In the morphogenic responses of the different types of explants used in P. albovittata and P. galioides, it was observed that they responded positively only in the treatments that included 0.02 mg l-1 IAA supplemented with 0.02 mg l-1 GA3 for P. albovittata, and 0.02 mg l-1 NAA supplemented with 0.02 mg l-1 GA3 for P. galiodes. In P. albovittata the shoots formation was observed without vitrification and in P. galioides organogenic nodules, shoots and roots formation (Table 1; Figure 2 e and Figure 3 a-b). Explants of leaves in P. albovittata and leaves with petioles in P. galioides, respectively, did not show morphogenic response.
Table 1. Number of shoot of Peperomia albovittata (leaves and petioles)and P. galioides (leaves with petiole and nodes), after 60 days of in vitro culture in media with different growth regulators.

Direct organogenesis was observed only in petioles of P. albovittata (Table 1; Figure 2 d) and it was started 15 days after the tissue were installed in vitro, while in P. galioides the process of indirect organogenesis was observed only in leaves explants (Figure 3 c).
In P. galioides, the shoots generated from the axillary buds of the nodes initiated a harmonic growth with the emergence of the apical bud and then the development of the root system, generating a new individual, the same one that was used, subsequently, for in vitro cloning and future germplasm conservation trials.
In other treatments tested with low concentrations of NAA and moderate concentrations of BAP, the formation of calluses was induced at various stages of development (+ to +++) only in leaves of P. albovittata and leaves with petiole in P. galioides, although the rate of phenolic browning was very high (Table 2). These results showed that the supplement of cytokinin BAP, at any concentration, was detrimental in the induction of indirect organogenic processes.
Table 2. Callus formation in Peperomia albovittata (leaves) and P. galioides (leaves with petiole), after 3-5 months of in vitro culture in media with different growth regulators. (Similar responses in both Peperomia species).
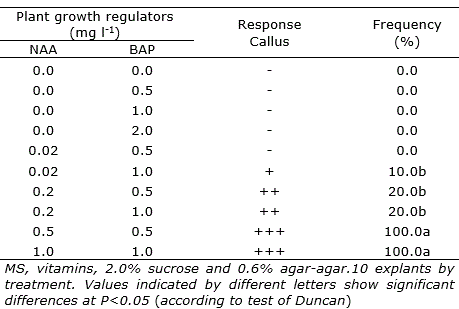
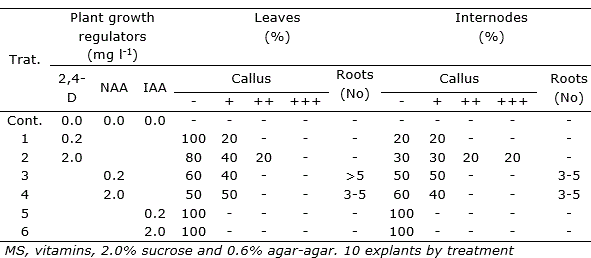
In P. galioides, callus induction was also observed in internodes (+++) and leaves (++), in the treatment supplemented with 2.0 mg l-1 2,4-D, while 0.2 and 2.0 mg l-1 NAA induced a moderate formation of calluses (+), both in internodes and leaves. IAA at concentrations of 0.2 and 2.0 mg l-1 did not induce callus (-) formation in the two explants tested (Table 3). In this species, direct organogenesis was observed in internodes and leaves, only in some interactions of NAA-BAP-GA3, at very low concentrations, reaching the highest number of regenerated shoots (7.4±1.8) in the interaction of 0.5 mg l-1 NAA - 0.02 mg l-1 BAP - 0.02 mg l-1 GA3, in internodes explants (Table 4).
Table 3. Effect of auxins (2,4-D, NAA and IAA) on callus induction and roots formation of Peperomia galioides, after three months of in vitro culture in media with different growth regulators.
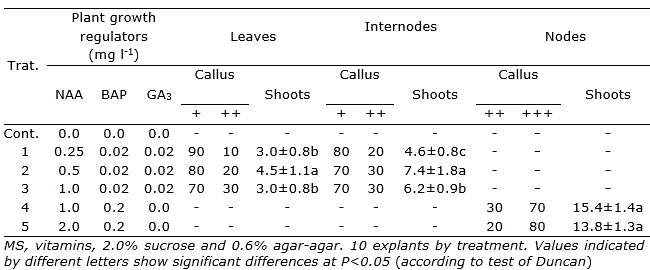
Additionally, high direct organogenesis rates of shoots (15.4±1.4) were observed in nodes of P. galioides in culture medium supplemented with 1.0 mg l-1 NAA - 0.2 mg l-1 BAP, although there was also a very high callus induction (70%, +++) (Table 4).
Table 4. Effect of NAA – BAP and NAA – BAP – GA3 on callus induction, shoots and roots formation of P. galioides, after three months of in vitro culture in media with different growth regulators.
DISCUSSION
Endophytic fungi are widely disseminated in healthy plant tissues constituting a unique micro-ecosystem, establishing a special relationship between the host plants and their endophytes, which can promote the biosynthesis of secondary metabolites of medicinal species (Jia et al., 2016). However, the endophytes can be severely affected by genetic factors, soil nutrients and environmental conditions of their hosts, although in compensation endophytes increase growth rate, resistance to pests and diseases, tolerance to several abiotic stresses, and biosynthesis of secondary compounds (Jia et al., 2016; Chen et al., 2016). On the other hand, although no studies on endophytic fungi have been reported in in vitro cultures of Peperomia (Piperaceae), in the Piperaceae family, specifically in Piper hispidum, isolated endophytes were identified belonging to 11 genera as Alternaria, Colletotrichum, Guignardia, and others, and these fungi can be useful for biological control of several pathogens and plant growth and development promotion (Orlandelli et al., 2012). Further experimental studies are necessary to detect and identify epiphytic and endophytes contaminants in in vitro culture of Peperomia species.
Vitrification is a frequent, serious, and complex problem in plant cell and tissue culture since that induce hyperhidricity and hypertrophy, it can affect the clonal propagation rate and culture vigour and can impede the successful transfer of in vitro plants to greenhouse conditions (Hammerschlag, 1986; Pasqualetto, 1990). The regulation of light and temperature in the growth room attenuated the rate of vitrification in both Peperomia species.
On the other hand, oxidative or phenolic browning is a frequent and severe problem in plant cell and tissue culture systems induced by the byosinthesis, accumulation and oxidation of phenolic compounds. This process resulting in reduced growth and lower or no regeneration rates that may lead to explant death, due to increased activity of the phenylalanine ammonia lyase enzyme (PAL) (Jones and Saxena, 2013). In this regard, studies on cell suspensions in species of the Piper genus, which together with Peperomia are the largest in the Piperaceae family, have shown the high degree of phenolic browning, both in the callus induction and in the establishment of cell suspensions (Delgado-Paredes et al., 2013), as observed in the in vitro culture of P. albovittata and P. galioides in this study. Alternatives for diminished the negative effects of this problems are required.
The scientific literature reports few studies on direct and indirect organogenesis in Peperomia species. The results of this study show that, for both P. albovittata and P. galioides, only the combinations between IAA-GA3 or NAA-GA3, at very low concentrations, induced the formation of adventitious buds, which is related to the results obtained by Ahmadabad and Bock (2010). This authors in Peperomia metallica and P. peduncularis, reported direct organogenesis from leaf discs, when low concentrations of GA3 were supplemented with IAA and BAP, resulting in 100% of regeneration efficiency. However, when IAA and zeatin (ZEA) were supplemented in different concentrations, no shoot regeneration was observed. They attributed the results to the effect of gibberellic acid in the process of dedifferentiation and cell redifferentiation in somatic cells of the leaves of both species of Peperomia. On the other hand, in P. obtusifolia, leaf segments were cultured on solidified MS medium supplemented with kinetin (KIN) at 0, 2.5, 5.0 and 10.0 mg l-1 or NAA at 0, 1.0, 2.5 and 5.0 mg l-1; observing that all the explants generated from leaf segments turned dark brown and died without shoots or callus formation (El-Naggar and Osman, 2014). In these two studies, the objective was to induce direct organogenesis, which was observed only in P. metallica and P. peduncularis. In another study, the percentage of successful shoots regenerated from nodal segments P. obtusifolia was high by increasing kinetin concentration, while several concentrations of NAA almost had no effect on percentage and number of shoot formation (El-Naggar y Osman, 2014). Besides, clonal propagation by direct organogenesis was induced in Peperomia pellucida, since the explants used were shoots tips and nodal explants with one or two axillary buds). These shoots were induced on MS medium augmented with 2.0 mg l-1 BAP and the further multiplied with 0.5 mg l-1 each of BAP and KIN (Shekhawat and Manokari, 2015).
The results in the present study confirmed previous finding about that cytokinins (BAP, KIN or ZEA) are useful in the morphogenic processes of clonal propagation of Peperomia, unless they are supplemented with as very low concentrations as have also been reported in the results for P. albovittata and P. galioides. However, it would be necessary to carry out additional tests with other cytokinins, such as 2iP, TDZ or Topolins. Likewise, a difference was observed in the morphogenic responses of both Peperomia species, where P. galioides showed a greater in vitro totipotency than P. albovittata. This specie was recalcitrant in its morphogenic responses when it was subjected to the same culture conditions as P. galioides.
CONCLUSIONS
Peperomia albovittata and P. galioides, two important ornamental and medicinal herbs used in folk medicine can be propagate by in vitro tissue culture techniques. The use of different explants for callus induction and direct organogenesis in culture medium supplemented with several combinations of growth regulators achieve the plants regeneration. However, some problems observed in the culture establishment, such as microbial contamination, vitrification and phenolic browning require more studies. The standardization of the results in other Peperomia species of the Peru flora will enable it propagation and germplasm conservation, seriously threatened by predation and destruction of their natural habitat.
ACKNOWLEDGMENTS
The authors are grateful to Prof. Abel Samamé-Caramutti for English improvement. The funder, Laboratorio General de Biotecnología – VRINV of the Universidad Nacional Pedro Ruiz Gallo (UNPRG), Lambayeque (Perú) no role in study design, data collection and analysis, decision of publish, or preparation of the manuscript. The funder pay the salary of Consuelo Rojas-Idrogo and Guillermo E. Delgado-Paredes and only registered the project in the Vicerrectorado de Investigación (VRINV-UNPRG). Matilde I. Olivera-Morante has no employment relationship with the university.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
Conceptualization CRI and GDP, Methodology CRI and MOM, writing - original draft CRI, writing- review & editing CRI and GDP.
REFERENCES
Ahmadabad M, Bock R (2010) Development of a highly responsive leaf-based regeneration system for Peperomia species. Turkish Journal of Botany 34: 329-334; doi:10.3906/bot-1001-309
Al-Madhagi WM, Hashim NM, Ali NAA, Alhadi AA, Halim SNA, Otham R (2018) Chemical profiling and biological activity of Peperomia blanda (Jacq.) Kunth. Peerj 6: e4839; doi:10.7717/peerj.4839. ecollection 2018
Aziba PI, Adedeji A, Ekor M, Adeyemi O (2001) Analgesic activity of Peperomia pellucida aerial parts in mice. Fitoterapia 72: 57-58
Bartels F (1965) Die Plastiden von Peperomia metallica (Plastidenzahlungen). Z Bot 52: 572-599
Bulychev AA, Andranov VK, Kurella GA, Litvin FF (1972) Micro-electrodes measurements of the transmembrane potential of chloroplasts and its photo induced changes. Nature 236: 175-177
Boufford DE (1982) Notes on Peperomia (Piperaceae) in the southeastern United States. Journal of teh Arnold Arboretum 63: 317-325
Brako L, Zarucchi JL (1993) Catálogo de las Angiospermas y Gimnospermas del Perú. Monographs in Systematic Botany from the Missouri Botanical Garden Vol 45. Missouri Botanical Garden, St. Louis MO
Chen L, Zhang QY, Jia M, Ming QL, Yue W, Rahman K, Qin LP, Han T (2016) Endophytic fungi with antitumor activities: their occurrence and anticancer compounds. Crit Rev Microbiol 42: 454-473; doi:10.3109/1040841X.2014.959892
Dahlstedt H (1900) Studien über süd - und central-amerikanische Peperomien mit besonderer Verücksichtigung der brasilianischen Sippen. Kongliga Svenska Vetenskaps-Akademiens handlinger 33: 1-218
de Fátima ABM, Dmitrieva EG, Franzotti EM, Antoniolli AR, Andrade MR, Marchioro M (2004) Anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae). Journal of Ethnopharmacology 91: 215-218; doi:10.1016/j.jeep.2003.12.030
Delgado-Paredes GE, Kato MJ, Rojas-Idrogo C (2013) Suspensiones celulares y producción de metabolitos secundarios en cultivos in vitro de Piper sp. Boletín Latinoamericano y del Caribe de Plantas medicinales y Aromáticas 12: 269-282
El-Naggar HM, Osman AR (2014) Micropropagation and organogenesis of Peperomia obtusifolia. Asian Journal of Crop Science 6: 58-66; doi:10.3923/ajcs.2014.58.66
Felippe LG, Baldoquei DC, Kato MJ, Bolzani Vda S, Guimarães EF, Cicarelli RM, Furlan M (2008) Trypanocidal tetrahydrofuran lignans from Peperomia blanda. Phytochemistry 69: 445-450; doi:10.1016/j.phytochem.2007.09.012
Frenzke L, Scheiris E, Pino G, Symmank L, Goetghebeur P, Neinhuis C, Wanke S, Samain MS (2015) A revised infrageneric classification of the genus Peperomia Ruis & Pav. (Piperaceae). Taxon 64: 424-444; doi:10.12705/643.4
Guimarães EF, Ichaso CLF, Costa CG (1984) Piperáceas. En: Reitz R (ed). Flora Ilustrada Catarinense 4. Peperomia, pp.33-136. CNPq, IBDF, HBR, Itajaí
Gutierrez YV, Yamaguchi LF, de Moraes MM, Jeffrey CS, Kato MJ (2016) Natural products from Peperomia: occurrence, biogenesis and bioactivity. Phytochemical Review 15: 1009-1033; doi:10.1007/s11101-016-9461-5
Hammerschlag FA (1986) Temperate fruits and nuts. En: Zimmerman RH, Griesbach RJ, Hammerschlag FA, Lawson RH (eds). Tissue Culture as a Plant Production System for Horticultural Crops, pp. 221-236. Martinus Nijhoff Publishers, Dordrecht
IBODA (2015) Base de Datos. Flora del Cono Sur. Instituto de Botánica Darwinion. Buenos Aires. Argentina. Disponible en: http://www2.darwin.edu.ar/. Consultado 04/mayo/2019
Jaramillo MA, Manos PS, Zimmer EA (2004) Phylogenetic relationships of the perianthless Piperales: reconstructing the evolution of floral development. International Journal of Plant Sciences 165: 403-416; doi: 10.1086/382803
Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LP (2016) A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Frontiers in Microbiology 7: 906; doi: 10.3389/fmicb.2016.00906
Jones AMP, Saxena PK (2013) Inhibition of phenilpropanoid biosynthesis in Artemisia annua L.: a novel approach to reduce oxidative browning in plant tissue culture. PLoS One 8(10): e76802; doi: 10.137/journal.pone.0076802
Ilyas S, Naz S, aslam F, Parveen Z, Ali A (2014) Chemical composition of essential oil from in vitro grown Peperomia obtusifolia through GC-MS. Pakistan Journal of Botany 46: 667-672
Loc NH, Bach TG, Kim TG, Yang MS (2010) Tissue culture and expression of Escherichia coli heat-labile enterotoxin B subunit in transgenic Peperomia pellucida. Protein Expression Purification 72: 82-86; doi: 10.1016/j.pep.2020.02.010
Mai P, Rossado A, Bonifacino JM, Waechter JL (2016) Taxonomic revision of Peperomia (Piperaceae) from Uruguay. Phytotaxa 244: 125-144; doi: 10.11646/phytotaxa.244.2.2
Malquichagua KJ, Delgado GE, Ripalda L, Max Young MC, Kato MJ (2005) Chromenes of polyketide origin from Peperomia villipetiola. Phytochemistry 66: 573-579; doi: 10.1016/j.phytochem.2005.01.003
Mathieu G, Samain M-S, Reynders M, Goetghebeur P (2008) Taxonomy of the Peperomia species (Piperaceae) with pseudo-epiphyllous inflorescences, including four new species. Botanical Journal of the Linnean Society 157: 177-196; doi: 10.1111/j.1095-8339.2008.00777.x
Mathieu G, Vergara-Rodríguez D, Krömer T, Karger DN (2015) Peperomia (Piperaceae) novelties from Veracruz State, Mexico. Phytotaxa 205: 268-276; doi: 10.11646/phytotaxa.205.4.6
Morandim-Giannetti AA, Pin AR, Pietro NAS, de Oliveira HC, Mendes-Giannini MJS, Alecio AC, Kato MJ, de Oliveira JE, Furlan M (2010) Composition and antifungal activity against Candida albicans, Candida parapsilosis, Candida krusei and Cryptococcus neoformans of essential oils from leaves of Piper and Peperomia species. Journal of Medicinal Plants Research 4: 1810-1814; doi: 10.5897/JMPR09.303
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497
Narayana V, Vasantha K, Rency RC, Maruthasalam A (2015) GC MS determination of bioactive components of Peperomia pellucida (L.) Kunth. Bioscience Discovery 6: 83-88
Narayana V, Vasantha K, Maruthasalam A (2018) In vitro evaluation of cytotoxic properties of Peperomia pellucida (L.) H.B.K. against human cancer cell lines. Bioscience Discovery 9: 344-355
Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OD, Prasad AK, Wengel J, Olsen CE, Boll PM (1997) Phytochemistry of the genus Piper.Phytochemistry 46: 597-673
Pasqualetto PL (1990) Vitrification in plant tissue culture. En: Rodríguez R, Tamés RS, Durzan DJ (eds). Plant Aging: Basic and Applied Approaches, pp. 133-137. Plenum Press, New York
Orlandelli RC, Alberto RN, Rubin Filho CJ, Pamphile JA (2012) Diversity of endophytic fungal community associated with Piper hispidum (Piperaceae) leaves. Genetics and Molecular Research 11: 1575-1585; doi: 10.4238/2012.May.22.7
Samain MS, Mathieu G, Vanderschaeve L, Wanke S, Neinhuis C, Goetghebeur P (2007) Nomenclature and typification of the subdivisional names of the genus Peperomia (Piperaceae). Taxon 56: 229-236; doi: 10.2307/25065756
Shekhawat MS, Manokari M (2015) Direct organogenesis for mass propagation of Peperomia pellucida L. and epiphytic medicinal herb. World Scientific News 23: 24-34
Sussa FV, Damatto SR, Alencar MM, Mazzilli BP, Silva PSC (2013) Natural radioactivity determination in samples of Peperomia pellucida commonly used as medicinal herb. Journal of Environmental Radioactivity 116: 148-151; doi: 10.1016/j.jenvrad.2012.09.012
Wanke S, Samain M, Vanderschaeve L, Mathieu G, Goetghebeur P, Neinhuis C (2006)
Phylogeny of the genus Peperomia (Piperaceae) inferred from the trnK/matK region (cpDNA). Plant Biology 8: 93-102; doi: 10.1055/s-1055-873060
Yuncker TG (1953) The Piperaceae of Argentina, Bolivia and Chile. Lilloa 27: 8-303
Yuncker TG (1974) The Piperaceae of Brazil III. Peperomia, taxa of uncertain status. Hoehnea 4: 71-413
Recibido: 28-01-2020
Aceptado: 23-03-2020
Copyright (c) 2020 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
