Artículo original
Biotecnología Vegetal Vol. 21, No. 1: 62-73, enero - marzo, 2021
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Effect of the inoculation of phosphate solubilizing microorganisms and arbuscular mycorrhizal fungi in Eugenia dysenterica plants grown on different substrates
Efecto de la inoculación de microorganismos solubilizadores de fosfato y hongos micorrícicos arbusculares en plantas de Eugenia dysenterica crecidas en diferentes sustratos
Juliana Silva Rodrigues Cabral1, https://orcid.org/0000-0001-9821-8718
Maria Gabriela Almeida Ceribeli2, https://orcid.org/0000-0002-5659-1538
Kerlley Cristina de Assis3, https://orcid.org/0000-0002-7365-6823
Fabiano Guimarães Silva2, https://orcid.org/0000-0003-4908-2265
Joana Junqueira Carneiro4, https://orcid.org/0000-0001-5684-1982
Tenille Ribeiro de Souza2*, https://orcid.org/0000-0002-9484-118X
Marco Aurélio Carbone Carneiro4, https://orcid.org/0000-0003-4349-3071
Edson Luiz Souchie2, https://orcid.org/0000-0003-2338-4812
1University of Rio Verde, Faculty of Agronomy. Fazenda Fontes do Saber -Campus Universitário. Rio Verde. Goiás. Brasil. Cx Postal 104 - CEP 75901-970.
2Federal Institute Goiano (IF Goiano) – Rio Verde Campus. Rodovia Sul Goiana km 01. Rio Verde. GO. Brasil. Cx P 66 CEP 75 901-970.
3Embrapa Temperate Climate. Rodovia BR 392, km 78. Pelotas. RS. Brasil. Cx Postal 403 96 010-971.
4Federal University of Lavras, Department of Soil Science / Laboratory of Soil Microbiology. Lavras. MG. Brasil. 37200-000.
*Corresponding author e-mail: tenillemicro@gmail.com
ABSTRACT
Phosphate solubilizing microorganisms (PSM) and arbuscular mycorrhizal fungi (AMF) represent alternative strategies to reduce the cost and increase the production of cagaita (Eugenia dysenterica) seedlings associated with different substrates. This study aimed to assess the effect of inoculating PSM and AMF in different substrates for cagaita (Eugenia dysenterica) culturing. The experiment was installed with a completely randomized design, using a 4 × 2 factorial scheme, with four inoculation treatments (PSM, AMF, PSM + AMF, and Control – absence of inoculation), and two soils substrates (pure sandy substrate and mixed substrate). The substrates and microorganism had influence in cagaita seedling growth. The pure substrate conferred better performance of plant growth parameters. In this substrate, cagaita seedlings had higher stem diameter, shoot fresh and dry weight, root volume and root dry weight. The cultivation of seedlings in pure substrate inoculated with AMF and PSM provided greater Mo content in the leaves. The higher root volume of cagaita seedlings was obtained with the inoculation of PSM. A sandy substrate seems to be the most adequate for the cultivation of cagaita seedlings. In addition, co-inoculation provides higher Fe, Mg, and K content in the leaves, as this work showed.
Keywords: cerrado, Claroideoglomus etunicatum, phosphorus
RESUMEN
Los microorganismos solubilizadores de fosfato (PSM) y los hongos micorrízicos arbusculares (HMA) representan estrategias alternativas para reducir el costo y aumentar la producción de plántulas de cagaita (Eugenia dysenterica) asociadas a diferentes sustratos. Este estudio tuvo como objetivo evaluar el efecto de la inoculación de PSM y HMA en diferentes sustratos para el cultivo de cagaita (Eugenia dysenterica). El experimento se instaló con un diseño completamente al azar, utilizando un esquema factorial 4 × 2, con cuatro tratamientos de inoculación (PSM, HMA, PSM + HMA y Control - ausencia de inoculación), y dos sustratos de suelo (sustrato arenoso puro y sustrato mixto). Los sustratos y microorganismos influyeron en el crecimiento de las plántulas de cagaita. El sustrato puro propició un mejor desempeño de los parámetros de crecimiento de las plantas. En este sustrato, las plántulas de cagaita presentaron mayor diámetro de tallo, masa fresca y seca de brotes, volumen de raíz y masa seca de la raíz. El cultivo de plántulas en sustrato puro inoculadas con HMA y PSM proporcionó mayor contenido de Mo en las hojas. El mayor volumen de raíces de plántulas de cagaita se obtuvo con la inoculación de PSM. Un sustrato arenoso parece ser el más adecuado para el cultivo de plántulas de cagaita. Además, la coinoculación proporciona un mayor contenido de Fe, Mg y K en las hojas, como mostró este trabajo.
Palabras clave: Cerrado, Claroideoglomus etunicatum, fósforo
INTRODUCTION
Arbuscular mycorrhizal fungi (AMF) stimulate plant growth under stress conditions in the soil, due to increased nutrient absorption, with the potential to minimize the adverse effects of seedling transplants, water deficiency, and attacks from pathogenic agents (Kohler et al., 2007; Ferreira et al., 2015; Diagne et al., 2020; Song et al., 2020). The use of these microorganisms can support the production, nutrition, and growth promotion of seedlings in nurseries, the establishment of seedlings in the field and under stress conditions, such as those associated with the cerrado biome (Brazilian savanna) (Anzanello et al., 2011; Coelho et al., 2012; Svenningsen et al., 2018).
Other soil microorganisms, in addition to AMF, have the ability to stimulate plant growth, through the production of phytohormones (gibberellin, auxin, and cytokinin) and phosphate solubilization. The inorganic phosphate solubilizing microorganisms (PSM) play an important role in the supply of phosphorus (P) to plants. The solubilizing action has been mainly associated with the production of organic acids (Barroso and Nahas, 2008; Alves and Silva, 2009). In the soil, PSM contribute to increase the P content in solution, which can be directly absorbed by roots or AMF hyphae that are symbionts to plants (Moreira and Siqueira, 2006; Zaidi and Khan, 2007). Phosphate solubilizing bacteria may also play an important role in the interactions between roots and AMF, acting as mycorrhiza helper bacteria. The inoculation of PSM is association with other beneficial soil microorganisms, may improve plant development (Narloch et al., 2002).
The cagaita (Eugenia dysenterica DC.), a Cerrado (Brazilian savannah) fruit specie, is a medium-height tree, with a trunk of approximately 4–10 m. As well as other native species, it has great importance due to the chemical composition and nutritional value, with levels of vitamins, minerals, phenolic compounds, and antioxidants (Chaves and Telles, 2006; Rodrigues et al., 2018).
The main use of this native plant is for food, but it has medical, pharmaceutical, ornamental, and honey production potential. The fruit is a round, large berry, with a fleshy and juicy pulp, and each fruit has 1–3 seeds. It is slightly acidic with a pleasant taste. The pulp of cagaita may be consumed in natura or in juice, jelly, liquor, as popsicle flavoring, and in sweets. Besides, cagaita tree is an ornamental plant, and its bark can be used in tanneries through cork extraction. Other application described are related to the employ in popular medicine by the laxative properties, and the tea made from the leaves has an antidiarrheal effect. The fruit is considered a source of vitamin C, with content of 26.84 g/100 g in green fruits and 27.46 g/100 g in ripe fruits, which is relatively high when compared with other native fruits of cerrado (Silva et al., 2005; Chaves and Telles, 2006; Rodrigues et al., 2018).
This species may be cultivated via seeds. Nevertheless, the germination is slow and uneven, taking up to sixty days for the process to stabilize. Additionally, the cagaita seeds are recalcitrant. They cannot be stored, since do not tolerate drying and/or freezing (Duarte et al., 2006; Martinotto et al., 2008). In this sense, the characteristics of substrates are very important.
For seedlings culture, different mixtures of substrate can be used, where good aeration allow better root development. The substrate should have good physical, chemical, and biological characteristics, allowing quick development of seedlings and abundant formation of the root system. A good water/air ratio, join to provide the required nutrients for plant growth, with homogeneous composition, will facilitate the management of seedlings and a cost compatible with the activity (Dias et al., 2007; Sobrinho et al., 2010).
In order to achieve the sustainable use of the different species and landscapes of the cerrado biome, it is necessary to improve the planning and management of the territory, to appropriately value and manage of these resources and to recover altered and degraded areas. The inoculation of different microorganisms can contribute to the development of plants favoring the use of the soil in relation to its chemical limitation. Studies on species native to the Cerrado (Brazilian savannah) are important, since these species differ with respect to their ability to form and benefit from symbiosis with microorganisms and growth in different substrates. Thus, the objective of this research was to assess the effect of inoculating PSM and AMF in different substrates for cagaita (Eugenia dysenterica) culturing.
MATERIALS AND METHODS
The experiment was conducted at the Laboratory of Agricultural Microbiology and Laboratory of Plant Tissue, at the Federal Institute Goiano (IF Goiano), Rio Verde Campus.
Plant material
The cagaita seeds were obtained from ripe fruits collected at the Fazenda Gameleira, at the municipality of Montes Claros de Goiás, GO, latitude (S) 16° 06′ 20″; longitude (W) 51° 17′ 11″.
Origin of soils-substrates
Two substrates were used for cagaita culture. It were named as pure substrate with sandy texture and mixed substrate, with clay loam texture.
For the pure substrate, the soil (typic quartzipsamment) was collected between 10 and 40 cm of depth at the Fazenda Gameleira, Montes Claros de Goiás, latitude (S) 16° 06′ 20″; longitude (W) 51° 17′ 11″, located at 644 m of altitude.
In addition, a mixed substrate was composed by a mixture of the Oxisol soil type collected in two localities: in a farm near to the municipality of Rio Preto located at 712 m of altitude and in the area of IF Goiano, Rio Verde Campus, located at 748 m of altitude. The samples were collected between 10 and 40 cm of depth and they were mixed in a ratio of 1:1 (v:v).
The substrates were not fertilized. Chemical and physical analyses were performed at the Laboratory of Soil Analysis, Federal University of Lavras (Table 1).
Table 1. Chemical and physical characteristics of pure and mixed substrate for Eugenia dysenterica.

Inoculation and implantation of the experiment
Five PSM were used, including three bacterial isolates (MU23, GU12, and TC1) and two fungal isolates (GIR and AR8), obtained previously from a rhizospheric soil of different plants according to Sylvester-Bradley et al. (1982). To obtain the PSM inoculum, a mixture of the five isolates was prepared. The bacterial and fungal suspensions were separately cultured in a 125 ml Erlenmeyer containing glucose and yeast extract broth, and they were incubated at 28 °C for 72 h. For direct enumeration of colony-forming units (CFU), a series of dilutions until 10-5 were performed, plating (three repetitions of dilutions 10-4 and 10-5) and incubating in Petri dishes at 28 °C for 72 h. The inoculants concentration were standardized to approx. 1x108 CFU ml-1.
The mycorrhizal inoculant used was Claroideoglomus etunicatum (Becker & Gerd.) C. Walker & A. Schüssler from the Laboratory of Soils at the UFG – Campus Jataí.
Culturing was conducted in 300 cm3 tubes. The AMF was inoculated in the planting hole below the seeds (3.3 g). The PSM were inoculated 62 days after emergence by pipetting 1 ml of inoculum broth (aprox. 1x108 CFU ml-1) at the base of each plant stem.
Assessment and trial design
Cagaita seedlings were collected at 216 days after sowing. The stem diameter (cm) was measured with the aid of a digital caliper. The fresh and dry weight of shoots and roots (g) (Guimarães et al., 2002) and the root volume (ml plant-1) (Simões et al., 2013) were determined. Besides, the content of phosphorus (P) available in the substrates (mg dm3) before and after the implantation of the experiment were quantified (Embrapa, 1979). Also, the nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), sodium (Na), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), cobalt (Co), molybdenum (Mo), and boron(B) content in the leaves were determined by the SOLOCRIA – Laboratório Agrocpecuário.
The symbiotic efficiency was calculated according to the formula: Symbiotic efficiency = [(weight of dry shoot of the inoculated plant - weight of dry shoot of the control)/ weight of dry shoot of the inoculated plant] × 100 (Plenchette et al., 1983).
The experiment had a completely randomized design, using a 4 × 2 factorial scheme, with four inoculation treatments (PSM, AMF, PSM + AMF, and Control – absence of inoculation) and two substrates (pure soil with sandy texture and mixed substrate with clay loam texture). Each treatment had 50 repetitions. The numeric data were evaluated statistically, using analysis of variance, grouping the means by the Scott-Knott test (at 5% of probability), and using the SISVAR software (Ferreira, 2011).
RESULTS
There was no effect in the interaction of the two factors, substrates × inoculation, for any of the growth variables, neither for the symbiotic efficiency and available P.
The inoculation factor had no influence in stem diameter, or fresh and dry shoot and root weight. On the other hand, for the root volume, it was observed an effect of substrates and inoculation factors, individually (p>0.05).
The pure substrate conferred better performance of plant growth parameters. In this substrate, cagaita seedlings had higher stem diameter, shoot fresh and dry weight, root volume and root dry weight (Figure 1, Figure 2).

Figure 1. Shoot weight and stem diameter of cagaita (Eugenia dysenterica) seedlings cultivated in two substrates. Averages followed by the same letter between substrates do not differ among themselves by the Scott-Knott test (5%).
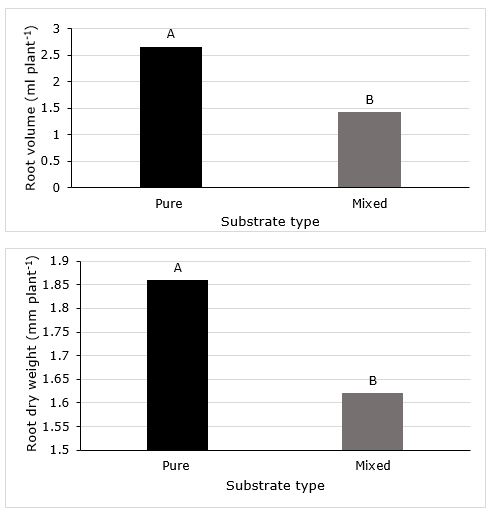
Figure 2.Root volume and root dry weight of cagaita (Eugenia dysenterica) seedlings cultivated in two substrates. Averages followed by the same letter between substrates do not differ among themselves by the Scott-Knott test (5%).
Comparing the inoculation treatments, the higher root volume of cagaita seedlings was obtained with the inoculation of PSM (Figure 3).

Figure 3. Root volume of cagaita (Eugenia dysenterica) seedlings inoculated with the arbuscular mycorrhizal fungi (AMF) Claroideoglomus etunicatum and phosphate solubilizing microorganisms (PSM). Averages followed by the same capital letter do not differ among themselves by the Scott-Knott test (5%).
However, inoculations of PSM, AMF, and PSM + AMF had negative symbiotic efficiency (Table 2), which means that the inoculation did not affect the shoot weight.
Table 2. Symbiotic efficiency of Claroideoglomus etunicatum and phosphate solubilizing microorganisms inoculated in cagaita (Eugenia dysenterica) seedlings cultivated in different substrates.

It was observed that the P content was higher in pure substrate before the implantation of the experiment. On the other hand, at the end of the experiment, the mixed substrates presented higher P content (Table 3).
Table 3. Phosphorus (P) content in two substrates inoculated with Claroideoglomus etunicatum and phosphate solubilizing microorganisms and cultivated with cagaita (Eugenia dysenterica) seedlings.

In the leaf analysis, for the nutrients potassium (K), zinc (Zn), and molybdenum (Mo), there was an interaction of the factors: substrate × inoculation treatments.
Using the pure substrate, the highest potassium value in leaves was observed for the control, without significantly differences with AMF+PSM. On the other hand, seedlings inoculated with PSM had a greater zinc content in the leaves. The treatments inoculated with AMF and PSM independently and co-inoculated obtained the greatest molybdenum content. In the case of the mixed substrate the content of Zn and Mo in leaves was similar in all treatments. K content as in pure substrate decreased with the inoculation of AMF and PSM (Table 4).
Table 4. Leaf content of potassium (K), zinc (Zn), and molybdenum (Mo) of cagaita (Eugenia dysenterica) seedlings cultivated in different substrates and inoculated with Claroideoglomus etunicatum (AMF) and phosphate solubilizing microorganisms (PSM).
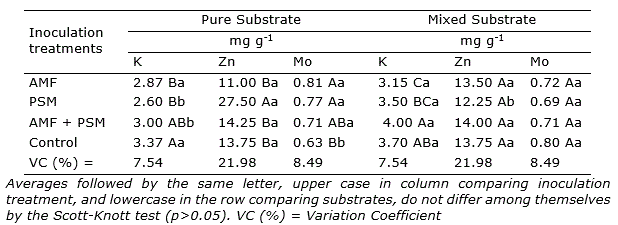
In the seedlings inoculated with AMF, there was not differences in absorption regardless of the substrate used. The inoculation of PSM enabled greater K content in the mixed substrate and Zn in the pure substrate, whereas for Mo there was no difference observed. The co-inoculation enabled greater K content in the mixture substrate, and there was no difference for Zn and Mo. In the control treatment, there was no difference for the K and Zn content between substrates, whereas greater Mo content was obtained using the mixed substrate (Table 4). There was no difference observed for nitrogen (N), phosphorus (P), sulfur (S), and sodium (Na).
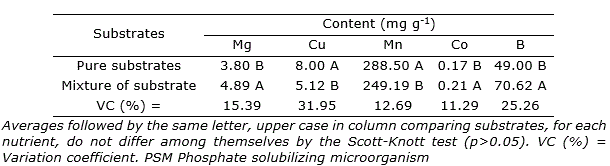
For the nutrients magnesium (Mg), copper (Cu), manganese (Mn), cobalt (Co), and boron (B) there was only influence from the substrates (p>0.05). The cagaita seedlings cultivated in mixed substrate accumulated greater leaf content of Mg, B, and Co. The pure substrate enabled greater accumulation of Cu and Mn (Table 5).
Table 5. Leaf content of magnesium (Mg), copper (Cu), manganese (Mn), cobalt (Co), and boron (B) of cagaita (Eugenia dysenterica) seedlings cultivated in different substrates and inoculated with Claroideoglomus etunicatum and PSM.
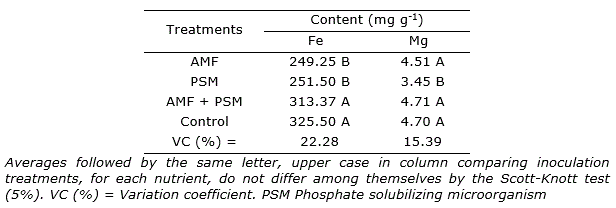
Iron (Fe) and magnesium (Mg) content of leaves were affected only by the inoculation treatments. The highest leaf content of Fe occurred when the seedlings were inoculated with AMF and PSM similar to the control treatment. The magnesium content of leaves was lower in the treatment inoculated with PSM (Table 6).
Table 6. Iron (Fe) and magnesium (Mg) content obtained from cagaita seedlings cultivated in different substrates and inoculated with Claroideoglomus etunicatum and PSM.
DISCUSSION
The two substrates had effect on the growth variables, with better performance in the pure one. Both substrates contained the same amount of organic matter (OM) (1.8 dag kg-1). But, others chemical characteristics (Table 1) revealed that the nutrients potassium (K), zinc (Zn), manganese (Mn), calcium (Ca), magnesium (Mg), and the effective CEC in pure soil are higher than in mixed substrate. It might has contributed to the better performance of the pure substrate, as well differences in physical composition. The sandy texture of the pure substrate could facilitate initial root development because of its low penetration resistance enabling higher growth of seedlings. Substrates with good porous space can affect water drainage and aeration balance, allowing higher growth of the root system along with a good nutrient availability (Sobrinho et al., 2010). Guimarães et al. (2011) and Alves et al. (2017) also reported the benefits of substrate with sand on the growth of seedlings of different tree species.
Related with that, Souza et al. (2001), obtained the higher shoot fresh and dry weight, and root volume of cagaita seedlings, when these were cultivated in substrate composed by soil + forest compost + vermiculite. Other authors such as Nietsche et al. (2004) reported that cultivation of cagaita in substrates containing soil + sand and soil + sand + manure resulted in higher averages compared to the commercial substrate Plugmix®. Carambola (Averrhoa carambola) seedlings had higher growth in soil + sand (Bastos et al., 2007), which indicated that production of seedlings in low investment substrates, considering the resistance to penetration, is satisfactory, supporting the results obtained in the present study conducted in pure soil with sandy texture (Table 1).
In the present work, Claroideoglomus etunicatum showed low symbiotic efficiency, that is, the fungus–plant–soil relation was not the best. In contrast, Santos et al. (2008) studied pioneering species such as Brazilian aroeira (Schinus terebenthifolius) and Jamaican nettletree (Trema micrantha) and two secondary species, the Brazilian açoita-cavalo (Luehea grandiflora) and sesbânia (Sesbania virgata), inoculated with different AMF, and concluded that none of the fungi isolated from the bauxite mining area was more efficient than C. etunicatum.
In contrast to the results obtained in this study, some authors reported the increase of nutrients N, P, and K in the leaves when seedlings of peach tree 'Okinawa' (Prunus persica (L.) Batsch), blueberry (Vaccinium spp.), and genipa (Genipa americana L.) were inoculated with C. etunicatum. This is in addition to the increase of Cu and Mn content of leaves in seedlings of genipa (Nunes et al., 2008; 2011; Soares et al., 2012; Farias et al., 2014). According to Pouyu-Rojas et al. (2006), the AMF have low specificity in colonization when compared to other symbiotic relations between plants and microorganisms.
Furthermore, the success of mycorrhizal inoculation depends on the fungus–plant–soil relations. The AMF species act differently depending on the host plants and soil conditions. Fruit plants inoculated with AMF may be favored by the symbiotic association, as long as the fungi species that is compatible with the plant has been inoculated. On the other hand, compatibility between species of AMF and plants is decisive for the beginning of the infection process and colonization of roots (Nunes et al., 2011; Torres et al., 2018).
None of the substrates used in this research were sterilized. So, the native microorganisms, including AMF and PSM, could be present in the original soils, that were collect near to the surface layer, where there are greater presence of microorganisms, and it could bias the inoculation effect in this case.
The PSM provide increased P supply to the plants by solubilizing unavailable forms of this nutrient (Zaidi and Khan, 2007; Magallon-Servín et al., 2020). Nevertheless, some factors can affect its growth, such as type of soil, plant species, and age of plant (Barroti et al., 2000). Certain combinations of soil and plants allow a better number of PSM and/or greater values of soluble P in a liquid environment (Souchie and Abboud, 2007; Magallon-Servín et al., 2020; Mehta et al., 2019).
The higher bioavailability of P through microorganisms have occurred in the mixed substrate (Table 3), however, it was not possible to observe the influence of this bioavailability in the improvement of the plant growth parameters or even in P leaves content. Nevertheless, the plants were removed from the soil at 216 days and, perhaps, if they had remained for a longer period in the system (substrate), the plant would have benefited through the absorption of this nutrient.
The low availability of other elements such as potassium (K), zinc (Zn), manganese (Mn), calcium (Ca), and magnesium (Mg), in addition to the low effective CEC and base saturation in the mixed substrates, may also have affected the low efficiency of this substrate in the growth of cagaita seedlings, compared to the pure substrate (Table 1).
The reduction of P in the pure substrate, on the other hand, may have occurred through the absorption of this nutrient by the plants, since in this substrate cagaita seedlings obtained greater root volume, enabling greater area of absorption of these in the soil and that of nutrients such as P, which is poorly mobile. The reduction of P in the pure substrate may also have occurred through the complexation with iron (Fe), present at a high concentration (476.7 mg dm-3), calcium (Ca), and magnesium (Mg) in this substrate (Table 1). The balanced level of the majority of nutrients in this substrate may have disguised the effect of low availability of P. Despite the values of symbiotic efficiency being negative, when inoculating the cagaita seedlings with PSM, higher root volume and Zn content were obtained. A greater Fe content in leaves was achieved with the co-inoculation AMF + PSM. The inoculation of AMF and AMF + PSM enabled greater Mg content, whereas higher K content was obtained with the co-inoculation using the mixed substrate. Moreover, the cultivation of seedlings in pure substrate inoculated with AMF and PSM enabled higher Mo content in leaves. For the Brasilian savannas species is important to know that some microorganisms can improve the capacity of absorption, because the soil is chemically poor and it can facilitate the seedlings survive and development.
CONCLUSIONS
The soil (typic quartzipsamment), the sandy pure substrate, is more adequate for the formation of cagaita seedlings. The co-inoculation AMF + PSM enabled greater Fe, Mg, and K content of leaves.
ACKNOWLEDGEMENTS
The CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior of Brazil and FAPEG, Fundação de Amparo à Pesquisa do Estado de Goiás, Brazil supported this research.The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
There are no conflicts of interest.
Author contributions
Conceptualization JSRC, MACC and ELS, Datacuration JSRC, FGS and ELS, Formal analysis JSRC, MGAC MACC and ELS, Investigation JSRC and ELS, Methodology JSRC, MGAC and KCA, Project administration ELS, Resources FGS and ELS, Supervision ELS, Validation JSRC, MGAC and ELS, Visualization JSRC, MGAC, KCA and ELS, Writing—original draft JSRC, JJC, TRS, MACC and ELS, Writing—review & editing JSRC, JJC, TRS, MACC and ELS.
REFERENCES
Alves L, Silva GN (2009) Produção de mudas de alface (Lactuca sativa L.) em presença de diferentes fontes fosfatadas e micro-organismos. Semina: Ciências Agrárias 30: 557-562
Alves MM, Alves EU, Araújo LRD, Lima MDL (2017) Substrate in the emergence and initial growth of seedlings of Caesalpinia pulcherrima. Ciência Rural 47(3): 1-5
Anzanello R, Souza PVD, Casamali B (2011) Fungos micorrízicos arbusculares (FMA) em porta-enxertos micropropagados de videira. Bragantia 70: 409-415
Barroti G, Nahas E (2000) População microbiana total e solubilizadora de fosfato em solo submetido a diferentes sistemas de cultivo. Pesquisa Agropecuária Brasileira 35: 2043-2050
Barroso CB, Nahas E (2008) Solubilização do fosfato de ferro em meio de cultura. Pesquisa Agropecuária Brasileira 43: 529-535
Bastos DC, Pio R, Scarpare JÁ, Libardi MN, Almeida LFP, Entelmann FA (2007) Diferentes substratos na produção de porta-enxertos de caramboleira. Ciência Agrotecnologia 31: 312-316
Chaves LJ, Telles MPC (2006) Frutas Nativas da Região Centro-Oeste do Brasil. Embrapa Recursos Genéticos e Biotecnologia, Brasilia
Coelho IR, Cavalcante UMT, Campos MADS, Silva FSB (2012) Uso de Fungos micorrízicos arbusculares (FMA) na promoção do crescimento de mudas de pinheira (Annona squamosa L.). Acta Botanica Brasilica 26: 933-937
Diagne N, Ngom M, Djighaly PI, Fall D, Hocher V, Svistoonoff S (2020) Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 12(10): 370
Dias TJ, Pereira WE, Sousa GG (2007) Fertilidade de substratos para mudas de mangabeira, contendo fibra de coco e adubados com fósforo. Acta Scientiarum Agronomy 29: 649-658
Duarte EF, Naves V, Borges JD, Guimarães NNR (2006) Germinação e vigor de sementes de Cagaita (Eugenia dysenterica MART. ex DC.) em função do tamanho e tipo de coleta. Pesquisa Agropecuária Tropical 36: 173-179
Embrapa - Empresa Brasileira De Pesquisa Agropecuária (1979) Manual de métodos de análises de solo. Serviço Nacional de Levantamento e Conservação de Solos, Rio de Janeiro
Farias DDH, Pinto, M A B, Carra, B, Schuch, MW, Souza PVDD (2014) Development of seedlings of blueberry inoculated arbuscular mycorrhizal fungi. Revista Brasileira Fruticultura 36: 655-663
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciência Agrotecnologia 35: 1039-1042
Ferreira GMDR, Melloni R, Silva LFDOD, Martins FB, Gonçalves ED (2015) Fungos micorrízicos arbusculares no desenvolvimento de mudas de oliveira (Olea europaea L.) cultivadas no sul de minas gerais. Revista Brasileira Ciência do Solo 39: 361-366
Guimarães IP, Coelho M, Benedito CP, Maia SSS, Nogueira CSR, Batista PF (2011) Efeito de diferentes substratos na emergência e vigor de plântulas de Mulungú. Bioscience Journal 27(6): 932-938
Guimarães VF, Echer MM, Minami k (2002) Métodos de produção de mudas, distribuição de matéria seca e produtividade de plantas de beterraba. Horticultura Brasileira 20: 505-509
Kohler J, Caravaca, F, Roldán A (2007) Interactions between a plant growth-promoting rhizobacterium, an AM fungus and a phosphate-solubilising fungus in the rhizosphere of Lactuca sativa. Applied Soil Ecology 35: 480-487
Magallon-Servín P, Antoun H, Taktek S, Bashan Y, de-Bashan L (2020) The maize mycorrhizosphere as a source for isolation of arbuscular mycorrhizae-compatible phosphate rock-solubilizing bacteria. Plant and Soil 451: 1-18; doi:10.1007/s11104-019-04226-3
Martinotto C, Paiva R, Soares FP, Santos BR, Nogueira RC (2008) Cagaiteira (Eugenia dysenterica DC. Myrtaceae). Boletim Técnico UFLA 78: 1-21
Mehta P, Sharma R, Putatunda C, Walia A (2019) Endophytic Fungi: Role in Phosphate Solubilization. In: Singh B (eds). Advances in Endophytic Fungal Research, pp. 183-209. Springer, Cham; doi:10.1007/978-3-030-03589-1_9
Moreira MFDS, Siqueira JO (2006) Microbiologia e Bioquímica do Solo, Capítulo 10 Micorrizas. Editora UFLA, Lavras
Narloch C, Oliveira VL, Anjos JT, Silva G (2002) Respostas da cultura do rabanete à inoculação de fungos solubilizadores de fosfatos. Pesquisa Agropecuária Brasileira 37: 841-845
Nietsche S, Gonçalves VD, Pereira MCT, Santos FA, Abreu SC, Mota WF (2004) Tamanho da semente e substratos na germinação e crescimento inicial de mudas de Cagaiteira.Ciência Agrotecnolgia 28: 1321-1325
Nunes JLDS, Souza, PVDD, Marodin, GAB, Fachinello, JC (2011) Development increase of 'Okinawa' peach rootstocks by indigenous arbuscular mycorrhizal fungi. Revista Ceres 58: 223-231
Nunes JLDS, Souza PVDD, Marodin GAB, Fachinello JC (2008) Inoculação de fungos micorrízicos arbusculares em porta-enxerto de pessegueiro cv Okinawa. Revista Brasileira de Fruticultura 30: 1100-1106
Plenchette C, Fortin JA, Furlan V (1983) Growth response of several plants species to mycorrhiza in a soil of moderate P fertility. I. Mycorrhizal dependency under field conditions. Plant and Soil 70: 199-209
Pouyu-Rojas E, Siqueira JO, Santos JGD (2006) Compatibilidade simbiótica de fungos micorrízicos arbusculares com espécies arbóreas tropicais. Revista Brasileira Ciência do Solo 30: 413-424
Rodrigues S, de Oliveira E, de Brito ES (Eds). (2018) Exotic Fruits Reference Guide. Academic Press, NY
Santos JGD, Siqueira JO, Moreira FMS (2008) Eficiência de fungos micorrízicos arbusculares isolados de solos de áreas de mineração de bauxita no crescimento inicial de espécies nativas. Revista Brasileira Ciência do Solo 32: 141-150
Silva MC, Santos GC, Nogueira PE, Munhoz CBR, Ramos AE (2005) 100 Árvores do cerrado Guia de Campo. Rede de Sementes do Cerrado, Brasília
Simões KS, Peixoto MFSP, Almeida AT, Ledo CAS, Peixoto C, Pereira FAC (2013) Água residuária de esgoto doméstico tratado na atividade microbiana do solo e crescimento da mamoneira. Revista Brasileira de Engenharia Agrícola e Ambiental 17: 518:523
Sylvester-Bradley R, Asakawa N, Latorraca S, Magalhães FMM, Oliveira LA, Pereira RM (1982) Levantamento quantitativo de microrganismos solubilizadores de fosfatos na rizosfera de gramíneas e leguminosas forrageiras na Amazônia. Acta Amazônia 12: 15-22
Soares, ACF, Sousa CDS, Garrido MDS, Lima FDS (2012) Arbuscular mycorrhizal fungi in the growth and nutrition of jenipapo fruit tree seedlings. Revista Ciência Agronômica 43: 47-54
Sobrinho SP, Luz PB, Silveira TTLS, Ramos DT, Neves LG, Barelli MAA (2010) Substratos na produção de mudas de três espécies arbóreas do cerrado. Revista Brasileira Ciências Agrárias 5: 238-243
Souchie EL, Abboud ACS (2007) Solubilização de fosfato por micro-organismos rizosféricos de genótipos de Guandu cultivados em diferentes classes de solo. Semina Ciências Agrárias 28: 11-18
Souza ERB, Carneiro IF, Naves RV, Borges JD, Leandro WM, Chaves LJ (2001) Emergência e crescimento de Cagaita (Eugenia dysenterica DC.) em função do tipo e do volume de substratos. Pesquisa Agropecuária Tropical 31: 89-95
Svenningsen NB, Watts-Williams SJ, Joner EJ, Battini F, Efthymiou A, Cruz-Paredes C, Jakobsen I (2018) Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. The ISME journal 12: 1296
Torres N, Goicoechea N, Zamarreño AM, Antolín MC (2018) Mycorrhizal symbiosis affects ABA metabolism during berry ripening in Vitis vinifera L. cv. Tempranillo grown under climate change scenarios. Plant science 274: 383-393
Zaidi A, Khan MS (2007) Stimulatory effects of dual inoculation with phosphate solubilising microorganisms and arbuscular mycorrhizal fungus on chickpea. Australian Journal of Experimental Agriculture 47: 1016-1022
Song Z, Bi Y, Zhang J, Gong Y, Yang H (2020) Arbuscular mycorrhizal fungi promote the growth of plants in the mining associated clay.Scientific reports 10(1): 1-9
Recibido: 16-06-2020
Aceptado: 17-09-2020
Copyright (c) 2021 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
