ARTÍCULO ORIGINAL
Biotecnología
Vegetal Vol. 14, No. 4: 215 - 221, octubre - diciembre, 2014
ISSN 2074-8647, RNPS: 2154 (Versión electrónica)
Instituto de Biotecnología de las Plantas. UCLV. MES.
Field resistance of selected potato clones to Early blight
Resistencia en campo de clones de papa al tizón temprano
Novisel Veitía*, Lourdes R. García, Idalmis Bermúdez-Caraballoso, Mayra Acosta- Suárez, Michel Leiva-Mora, Damaris Torres, Carlos Romero, Pedro Orellana. *Author for correspondence
Instituto de Biotecnología de las Plantas, Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba. e-mail: novisel@ibp.co.cu
ABSTRACT
Six potato clones, selected in vitro for their resistance to Alternaria solani Sor. culture filtrates, were evaluated for their field response to early blight infection. Field screening were performance under artificial inoculation and natural conditions. Early blight response was evaluated based on lesion size, disease severity, and area under disease progress curve (AUDPC). One clone displayed reduced lesion area (0.35 cm2) and AUDPC values compared to cv. `Desirée' (susceptible control) (0.58 cm2) but those values were higher than that of the resistant control Solanum chacoense `PI 275136' (0.14 cm2). On the other hand, no differences in lesion number were detected between the susceptible control and the selected clones. This variable showed values between 21.82 and 23.87 lesions in two leaves per plant. Although early blight resistance in potato is generally associated to late maturity, the mutant IBP-27 displayed increased resistance to early blight with medium-late maturity. The six clones presented medium-early to medium-late maturity, similar to parental cv. `Desirée' (vegetative cycle ranging from 90 to 110 days). One clone was found to have higher levels of resistant to early blight than cv. `Desirée' but lower than the levels of the resistant control S. chacoense. The resistance in this clone was characterized by the reduction in lesion area, disease severity, and AUDPC values in both artificial inoculation and natural infection screening.
Keywords: Alternaria solani, components of resistance, Solanum tuberosum
RESUMEN
Seis clones de papa, seleccionados por su resistencia in vitro al filtrado de cultivo de Alternaria solani Sor. se evaluaron para determinar su respuesta de campo a la infección por el tizón temprano. Los ensayos de campo se realizaron mediante inoculación artificial y en condiciones naturales. La respuesta al tizón temprano se evaluó en función del tamaño de la lesión, la severidad de la enfermedad y el área bajo la curva de progreso de la enfermedad (AUDPC). Un clon mostró menor área de la lesión (0.35 cm2) y valores de AUDPC en comparación con el cv. `Desirée' (control susceptible) (0.58 cm2) pero esos valores fueron superiores a los del control resistente Solanum chacoense `PI 275136' (0.14 cm2). Por otro lado, no se detectaron diferencias en el número de lesiones entre el control susceptible y los clones seleccionados. Esta variable mostró valores entre 21.82 y 23.87 lesiones en dos hojas por planta. Aunque la resistencia al tizón temprano de la papa se asocia generalmente a la madurez tardía, el mutante IBP-27 mostró una mayor resistencia al tizón temprano con madurez media. Los seis clones presentaron una madurez a media temprana a media tardía, similar al cv. progenitor `Desirée' (ciclo vegetativo que van desde 90 a 110 días). Se encontró un clon con niveles más altos de resistencia al tizón temprano que el cv. `Desirée', pero inferiores a los niveles del control resistente S. chacoense. La resistencia en este clon se caracterizó por la reducción en el área de la lesión, la severidad de la enfermedad, y los valores AUDPC tanto en la inoculación artificial como en la infección natural.
Palabras clave: Alternaria solani, componentes de resistencia, Solanum tuberosum
Abbreviations: EB- Early blight, AUDPC- area under disease progress curve
INTRODUCTION
Early blight (EB) caused by Alternaria solani Sorauer is one of the most important foliar diseases of potato (Solanum tuberosum L.) (Pelletier and Fry 1990; van der Waals et al., 2003). Fungicide application is the main control practice used worldwide (Gent and Schwartz, 2003). However, an increase in fungicide insensitivity in A. solani populations has been reported (Pasche et al., 2004). Genetic resistance offers an attractive alternative to chemical control because it reduces production costs and reduces the negative impact of fungicides in the environment (Shtienberg et al., 1995).
Adequate levels of EB resistance are not known within cultivated potato species (Boiteux et al., 1995; Cassells and Kowalski, 1998). However, sources of EB resistance have been identified in accessions of wild Solanum species. However, natural crossing barriers are present making difficulty the introgression of this resistance from wild species accessions into commercial varieties (Jansky, 2006). For that reason, selection of EB resistant cultivars with attractive commercial characteristics is not easily achieved (Cassells and Kowalski, 1998).
In vitro mutation treatment to induce variability followed by in vitro selection for EB resistance is considered an important tool in potato breeding (Cassells and Kowalski, 1998). Alternaria solani culture filtrates could be used to select EB resistant genotypes from susceptible ones (Lynch et al., 1991; Martinez and Sinclair, 1994). Lynch et al. (1991) reported that results of culture filtrate assays did not correspond with the results from greenhouse or field resistance screenings. In contrast, Martinez and Sinclair (1994) described a high correlation between in vitro selection plants with A. solani culture filtrates and in vivo response to EB infection in greenhouse. However, part of the studies published were mainly focused on the evaluation of the effect of culture filtrates on either cells or tissues cultured in vitro and comparing them with the in vivo response of plants in greenhouse (van den Bulk, 1991).
Pelletier and Fry (1989) and Boiteux et al. (1995) characterized the response of different potato varieties and clones based on components of resistance. However, to the best of our knowledge, field characterization of early blight response of potato clones generated from irradiated tissue with increased levels of resistance to A. solani culture filtrate is not described yet.
We previously reported the selection of six potato clones, derived from the early blight susceptible cv. Desirée, with lower levels of infection under natural infection in both greenhouse and field conditions (Veitía et al., 2007). The six clones were selected from a population of 1 000 putative mutants, obtained from irradiated callus cultures which were inoculated in vitro with culture filtrates of A. solani. In the present study, we describe the assessment of EB field resistance of the six potato clones under both artificial and natural inoculums conditions.
MATERIALS AND METHODS
Plant material
Six potato clones (IBP-27, IBP-30, IBP-38, IBP-93, IBP-101 and IBP-107) were used in this study. They were selected from irradiated callus cultures of the susceptible cv. `Desirée' inoculated with A. solani culture filtrates (Veitía et al., 2007). Two sets of field experiments were performed to assess the EB resistance of the mutant clones. Cv. `Desirée' was used as the susceptible control (Lorenzo et al., 1992) and wild potato accession Solanum chacoense BIT type `PI 275136' as the resistant control (Estévez et al., 1993). One experiment was conducted with artificial inoculation of the pathogen using mycelial suspensions and a second experiment was carried out under natural inoculum conditions.
Both experiments were planted in a field with a red ferralitic soil under irrigation at Pedro Lantigua Experimental Station (Remedios, Cuba). Tubers with 35-45mm diameter originated from in vitro cultured plants were used as seeds.
Artificial inoculation
Tubers of the six potato clones as well as of the susceptible and resistant controls were planted (for each experiments) in a randomized complete block design with four replicates. Each block was divided into eight plots corresponding to the six clones plus the susceptible and resistant controls (128 experimental plots in total). Each plot consisted of five rows with six plants (30 plants per plot). Seed tuber were planted 0.20 m apart within and 0.90 m between rows. Fertilizer was applied at the rate of 1.5 t ha-1 in NPK formulation of 9:17:13. No fungicide was applied to control EB incidence. The 12 central plants of each plot were considered for evaluation.
An A. solani isolate (CCIBP-VAs4) obtained from infected potato leaves in Las Antillas, Villa Clara, Cuba was used for artificial inoculation. Inoculum preparation was done as described by Leiva-Mora et al. (2006).
Plants (35 days after planting) were inoculated with a mycelial suspension. The inoculation was performed early in the morning by spraying until run off with a suspension containing 2x105 mycelia fragments per milliliter supplemented with 1% (w/v) gelatin.
Two leaves of the middle section of the plants were evaluated weekly post inoculation for lesion number and diameter. The lesion diameter was measured with a ruler and the lesion area was estimated using the ellipse area formula (A= (a/2*b/2)* ð), where, a is the length, b the width, and ð=3.14.
EB progress was assessed weekly using the scale described by Horsfall and Barrat (1945). Disease severity was estimated using the Towsend and Heuberger formula (where n is the total plants per degree of the scale, v is the rating of diagrammatic scale, i is the highest rating of diagrammatic scale, and N is the total plants evaluated).
Disease severity values were plotted against time and the area under disease progress curve (AUDPC) for each genotype was calculated using the mean disease severity values of each genotype (Jerger and Viljanen-Rollinson, 2001). Also, the vegetative cycle of plants was evaluated through tuber maturity.
Natural infection
This experiment
was performed using the same design as previously described, except that four
guard rows of cv. `Desirée' were used around the entire the experimental
field
aiming to provide a source of natural inoculum of A. solani conidia.
Reaction to EB was assessed weekly starting from 45 days after planting. The visual estimation of disease damage (using the Horsfall-Barratt grading scale). Disease severity and AUDPC were estimated as described above. The vegetative cycle of plants, tuber yield and number were also evaluated.
Statistical analysis
The Statistical Package for Social science (version 13.0) for Windows was used to analyze the lesion size, disease severity and AUDPC data. KruskalWallis non-parametric test was applied in the analyses.
RESULTS
Artificial inoculation
At seven days post inoculation (DPI), the lesions number was higher in all mutant clones (lesion number mean of 24.6-29.5) and in the susceptible control cv. `Desirée' (lesion number mean of 29.3) when compared with the resistant control S. chacoense (lesion number mean 5.2) (Table 1).
The lesion area parameter allowed better discrimination of the mutant clone from the susceptible control (cv. `Desirée'). One clone, IBP-27, displayed an average lesion area (cm2) lower than the susceptible control but higher than the resistant control (S. chacoense) (Table 1).
The lesions in the S. chacoense accession were restricted to the infection sites with no expansion throughout the evaluation period. IBP-27 clone reaction was inferior to that observed in the resistant control, but lesion expansion was more restricted to the infection site than in the other clones as well as in cv. `Desirée' (Figure 1).
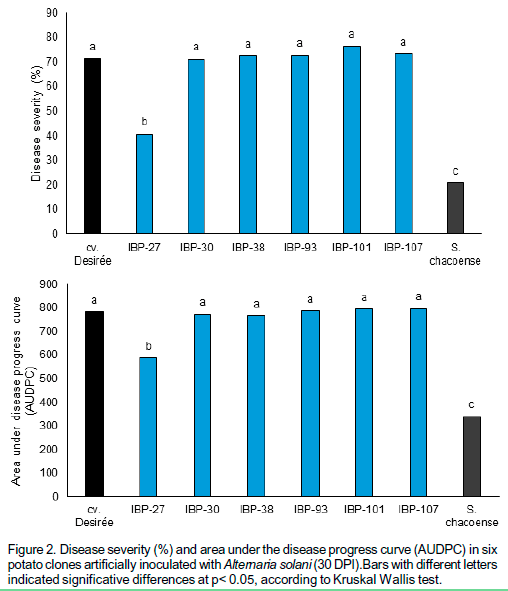
Five out of six potato clones evaluated had diseases severity indexes (%) similar to the susceptible control, but IBP-27 clone displayed a disease severity index significantly lower than cv. `Desirée' and significantly higher than S. chacoense (Figure 2).
The six clones presented medium-early to medium-late maturity, similar to the parental cv. `Desirée', with a vegetative cycle ranging from 90 to 110 days.
Natural infection
Under natural infection conditions, IBP 27 clone also displayed higher levels of EB resistance and with lower disease severity (%) when compared to the susceptible control and the other five mutant clones (Figure 3). Once more, the resistant control showed lower disease severity values (%) than IBP-27clone.
The clones IBP-30, IBP-38, IBP-93, IBP-101 and IBP-107 showed AUDPC values similar to that of the susceptible control cv. `Desirée'. Only IBP-27, presented lower AUDPC values than cv. Desirée, but higher than resistant control (Figure 2).
IBP-27 showed similar response in both experimental conditions (artificial inoculation and natural infection screenings) with lower disease severity and AUDPC values compared to the susceptible control, although higher than in the resistant control. The six potato clones evaluated presented a similar vegetative cycle to the parental cv. `Desirée'.
DISCUSSION
In field trial under artificial inoculation one mutant clone (IBP-27) expressed improved levels of EB resistance. This clone was characterized as having lower lesion area values than the susceptible control and the other five mutant clones (Table 1). Similar results were described by Pelletier and Fry (1989) for a resistant potato cultivar `Rosa'. These authors informed that the resistant response which delays tissue colonization after fungi penetration plays an important role in potato-Alternaria solani interaction. However, the smallest lesion area values were observed in the resistant control S. chacoense, confirming that this accession of this wild potato relative as being an excellent source of resistance genes to EB. This resistance can be introgressed into the cultivated S. tuberosum using 4x-2x (using synaptic mutants with 2n-pollen formation by first division restitution) breeding strategy (Peloquin et al., 1999; Buso et al., 2000).
It is interesting to note that no differences between lesion number of the susceptible control and the IBP-27 clone were detected. But, lower lesion number values in S. chacoense suggest that activation of resistant mechanisms might occur after the penetration stage.
For the parameter disease severity (%) and AUDPC the IBP-27 clone was found to have lower values than the susceptible control under both artificial inoculation (Figures 2) and natural infection (Figures 3). The others mutant clones had presented diseases severity similar to that of the susceptible control. The results are in agreement with a recently reported study involving this group of potato clones (Veitía et al., 2007). In that study, the IBP-27 clone was the only one identified displaying a significantly decreased early blight rating of affectation under natural infection under field conditions and had tuber yield similar to the susceptible control (cv. `Desirée').
As described by Johanson and Thurston (1990) early blight epidemics initially progresses slowly, but it accelerates with the plant maturity. However, this increase in IBP-27 was less, as shown, by lower values of disease severity (%) and AUDPC at 30 DPI. Several authors described the use of diseases severity integrated over time as AUDPC to characterize cultivars according to the resistance level (Boiteux et al., 1995; Christ and Haynes, 2001; Liatukas and Ruzgas, 2011; Duarte et al., 2014) since it allows a better description of the resistance response in epidemiological terms (Pandey et al., 2003).
No differences were found in the maturity cycle between the mutant clones and the parental cv. `Desirée'. The identification of one potato clone with a reduced lesion area, an AUDPC value and identical maturity rating to cv. `Desirée' suggests the existence of some mechanisms that acts regardless of the physiological maturity of the tissue. The association of late maturity with EB resistance has been documented for potato (Johanson and Thurston, 1990). These authors observed that late maturity clones were the most resistant ones but, not all early maturing clones were susceptible to EB pathogen. Also, they found one medium maturity clone that retained a resistant level to EB. In potato about half of the genotypic variation for EB resistance is also linked to maturity (Chaerani and Voorrips, 2006). However, different responses between potato cultivars with the same type of maturity had been found. For example, Boiteux et al. (1995) found one clone with high commercial production, lower values of AUDPC and early maturity.
The phytotoxicity of A. solani culture filtrates have been demonstrated in biotest on potato and tomato plants (Lynch et al., 1991; Maiero et al., 1991; Martinez and Sinclair, 1994). Most of secondary metabolites contained in A. solani culture filtrates are non host selective phytotoxins that interact with different target of the physiology of plants (Chaerani and Voorips, 2006). They act as virulence factor and intensify disease symptoms severity (Thommas, 2003). In the present work, A. solani culture filtrates (CCIBP-VAs4) could be a factor of virulence because, a potato mutant selected in vitro showed improved field performance to EB which was characterized by a reduction in the lesion area, disease severity, and AUDPC values when compared with the susceptible control cv. `Desirée'.
In summary, our studies allowed to characterize the resistance of potato clones selected in vitro via A. solani culture filtrates. Field evaluation of the selected clones allowed better characterization of EB resistance in the clones. Further studies are needed to extend our knowledge about other mechanisms which may be involved in the resistance to EB. These studies must be focused on the investigations on the role of A. solani metabolites in triggering resistance manifested as reduced lesion area.
ACKNOWLEGMENTS
We are very grateful to Dr. L.S. Boiteux and Dr. R. Chaerani for helpful comments and critical reading of the manuscript.
REFERENCES
Boiteux LS, Reifschneider FJB, Fonseca MEN, Buso JA (1995) Search for sources of early blight (Alternaria solani) field resistance not associated with vegetative late maturity in tetraploid potato germplasm. Euphytica 83: 63-70
Buso JA, Boiteux LS, Peloquin SJ (2000) Evaluation under long-day conditions of 4x-2x progenies from crosses between 4x potato cultivars and 2x haploid Tuberosum-Solanum chacoense hybrids. Annals of Applied Biology 136: 35-40
Cassells AC, Kowalski B (1998) Strategies for the evaluation of variation as source of resistance to early blight and late blight of potato. In: Khurana P, Chandra R, Mahesh D (eds). Comprehensive potato technology, pp 50-60. Malhotra Publishing House. New Delhi
Chaerani R, Voorrips RE (2006) Tomato early blight (Alternaria solani): the pathogen, genetics, and breeding for resistance. J Gen Plant Pathol 72: 335347
Biotecnología Vegetal Vol. 14, No. 4, 2014 221Christ BJ, Haynes KG (2001) Inheritance to early blight disease in a diploid potato population. Plant Breed 120: 169-172
Duarte S S, Zambolim Laércio, Rodrigues Fabrício, Paul Pierce A, Pádua Joaquim G, Ribeiro José I, Júnior Antonio F N, Rosado André W C (2014) Field resistance of potato cultivars to foliar early blight and its relationship with foliage maturity and tuber skin types. Tropical Plant Pathology 39(4): 294-306
Estévez A, González ME, Hernández MM, Ortiz U, Arzuaga J, Cordero M, González A, Martínez M, Lorenzo P (1993) Caracterización de un grupo de especies silvestres en papa. Cultivos tropicales 14(1): 80-85
Horsfall JF, Barrat RW (1945) An improved grading system for measuring plant diseases. Phytopathology 35: 655
Jansky S (2006) Overcoming hybridization barriers in potato. Plant Breed 125: 1-12
Jerger MJ, Viljanen-Rollinson SLH (2001) The use of area under the diseases-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theor. Appl. Gen. 102: 32-40
Johanson A, Thurston HD (1990) The effect of cultivar maturity on the resistance of potato to early blight caused by Alternaria solani. Am Potato J 67: 615-623
Liatukas Z, Ruzgas V (2011) Resistance of European winter wheat cultivars to spot blotch at juvenile growth stages. Phytopathol. Mediterr. 50: 350-358
Lorenzo P, Ramos M, Hernández MM (1992) Extracción de la toxina producida por el hongo Alternaria alternata. Cultivos Tropicales 13: 67-69
Lynch DR, Coleman, MC and Lyon GD (1991) Effect of Alternaria solani culture filtrate on adventitios shoot regeneration in potato. Plant Cell Reports 9: 607-611
Martinez PR, Sinclair M (1994) Selection in vitro of resistance to Early Blight (Alternaria solani Sorauer.) in Creole Potatoes (Solanum phureja Junz). Fitopatol Colomb 18: 90100
Maiero M, Bean GA, Ng TJ (1991) Toxin production by Alternaria solani and its related phytotoxicity to tomato breeding clones. Phytopathology 81: 1030-1033
Pandey KK, Pandey PK, Kallo G, Banerjee MK (2003) Resistance to early blight of tomato with respect to various parameters of disease epidemics. Journal Gen Plant Pathol 69: 364-371
Pasche JS, Wharam CM, Gudmestatad NC (2004) Shift in sensitivity of Alternaria solani response to Q(o)I fungicides. Plant Dis 88: 181-187
Pelletier JR, Fry WE (1989) Characterization of resistance to early blight in three potato cultivars: Incubation period, lesion expansion rate, and spore production. Phytopathology 79: 511-517
Pelletier JR, Fry WE (1990) Characterization of resistance to early blight in three potato cultivars: Receptivity. Phytopathology 80: 366-370
Peloquin SJ, Boiteux LS, Carputo D (1999) Meiotic mutants in potato: Valuable variants. Genetics 153: 1493-1499
van den Bulk RW (1991) Application of cell and tissue culture an in vitro selection for disease resistance breeding- a review. Euphytica 56: 269-285
van der Waals JE, Denner FDN, van Rij N, Korsten L (2003) Evaluation ol plant-plus, a decision support system for control early blight on potatoes in South Africa. Crop Prot. 22: 821-828
Veitía N, Kowalski B, Rodríguez LG, Caraballoso IB, Suárez MA, Pérez PO, Quintana CR. González N, Ramos RQ (2007) In vitro and ex vitro selection of potato plantlets for resistance to early blight. Phytophathology 155: 582-586
Recibido: 21-01-2014
Aceptado:
16-07-2014
Copyright (c) 2016 Biotecnología Vegetal
![]()
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.