Artículo Científico Biotecnología Vegetal Vol. 7, No. 3: 149 - 154, julio-septiembre, 2007
ISSN 1609-1841 (Versión impresa) ISSN 2074-8647 (Versión electrónica)
Effect of inoculum density and immersion time on the production of potato microtubers in temporary immersion systems and field studies
Naivy Pérez-Alonso*, Elio Jiménez, Manuel de Feria, Alina Capote, Raúl Barbón, Elisa Quiala, Maité Chávez. *Autor para correspondencia
Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas, Carretera a Camajuaní km 5.5 Santa Clara, Villa Clara. Cuba, CP 54 830. e-mail: naivy@ibp.co.cu
ABSTRACT
Microtubers can be easily induced in vitro, however so far their small size and low yield have restricted their use as propagules. Temporary immersion systems were used to produce potato microtubers and the effect of culture parameters as inoculum density and time of immersion on microtuberization were studied. When the inoculum density was increased from 60 to 90 explants per TIS no differences were observed regarding the number of microtubers per plant. However, fresh weight of the microtubers was superior with 60 explants (164.70g) compared with 47g obtained with 90 explants per TIS and the diameter of microtubers was affected adversely too. Immersion times of 2 minutes increased the number of microtubers per plant, as well as their size and quality, compared to immersion times of 30 minutes. In field studies plants derived from microtubers produced minitubers with higher diameter and fresh weight than plantlets from in vitro culture, with minituber numbers per plant similar in both treatments. The feasibility of developing a protocol for microtuber production in TIS and their direct planting into open field for the commercial production of potato minituber seed was demonstrated.
Key words: explant density, liquid culture, microtuberization, semi-automation, Solanum tuberosum L.
RESUMEN
Los microtubérculos pueden ser inducidos in vitro fácilmente, sin embargo su pequeño tamaño y bajo rendimiento han restringido su uso como propágulos. Se emplearon sistemas de inmersión temporal para la producción de microtubérculos de papa y se estudiaron parámetros de cultivo como la densidad de inóculo y el tiempo de inmersión en la microtuberización. Cuando la densidad de inóculo se incrementó de 60 a 90 explantes por sistema no se observaron diferencias en el número de microtubérculos por planta. Sin embargo, el peso fresco de los microtubérculos fue superior con 60 explantes (164.70g) con respecto a 47g que se obtuvieron con 90 explantes por SIT y el diámetro de los microtubérculos también se afectó. En el tratamiento con dos minutos de inmersión se logró un incremento en el número de microtubérculos por planta así como su tamaño y calidad comparado con el tratamiento de 30 minutos. En los estudios en campo las plantas obtenidas de microtubérculos produjeron minitubérculos con mayor diámetro y peso fresco que las plantas cultivadas in vitro, con similar número de minitubérculos por planta en ambos tratamientos. Se demostró la posibilidad de desarrollar un protocolo para la producción de microtubérculos de papa en sistemas de inmersión temporal que pueden ser plantados directamente en condiciones de campo para la producción comercial de semilla de papa.
Palabras clave: cultivo líquido, densidad de explantes, minitubérculos, semi-automatización, Solanum tuberosum L.
INTRODUCTION
Production of potato (Solanum tuberosum L.) microtubers (small tubers produced in vitro) represents a potential novel way for potato seed production from microplant stock, compared to some limitations present with the use of in vitro plantlets, which are difficult to store, bulky and delicate and difficult to handle and require an additional greenhouse acclimatization stage (Coleman et al., 2001; Pruski et al., 2002).
Microtubers are easier to handle and store, and feasible for automated plantation (Struik and
Wiersema, 1999; Coleman et al., 2001). Duplessis et al. (2000) reported that plants derived from microtubers are normal and strong and can be used in the production of original seed. However, on semi-solid culture media microtuberization has generally been characterized by the low yield of tubers (1-1.5 tubers/plant) and small tuber size which limits the success rate of direct transplant to field conditions (Jiménez et al., 1999; Struik and Wiersema, 1999; Lê, 1999).
In order to improve tuber quality and number of microtubers produced per plant several semi-automated systems have been developed, based on temporary immersion as well as bioreactors, with and without forced ventilation (Akita and Ohta, 1998; Ziv et al. 1998; Teisson and Alvard, 1999; Jiménez et al., 1999; Yu et al. 2000). These semi-automated systems also allow the reduction of intensive manual handling and hence increase productivity and decrease the costs of production (Etienne and Berthouly, 2002).
A temporary immersion system (TIS) for potato microtuber production was designed by Jimenez et al. (1999) and several advantages of this technique compared to semisolid cultures were demonstrated, such as three fold increase in shoot length, increased number of internodes per plant and improved plant vigor in all the cultivars tested. They observed that, in TIS, tuberization is not restricted to specific regions (nodes) of the plants and size and weight of the microtubers produced were higher than on semi-solid media.
However, the influence of culture parameters such as inoculum density and immersion times on nutrient assimilation as well as on the occurrence of hyperhydricity has so far not been extensively investigated. The present paper studies the effects of these culture parameters (inoculum density and time of immersion) on potato microtuberization in liquid culture medium and the performance of propagules obtained by temporary immersion techniques in field conditions.
MATERIALS AND METHODS
Plant material and culture conditions
Potato plantlets (Solanum tuberosum L. cv Atlantic) were established from apical meristems of sprouted tubers and multiplied as single-node explants on MS medium (Murashige and Skoog, 1962) with 0.5 mg.l-1 thiamine HCl, 100 mg l-1 myo-inositol, 20 g l-1 sucrose and gelified with 7.0 g l-1 agar (Type A, Sigma). Shoots were subcultured every 21 days. For tuber induction and development of microtubers a similar culture medium, but supplemented with 80 g l-1 sucrose was used. The pH of the medium was adjusted to 5.8 before autoclaving.
A temporary immersion system (TIS) as described by Jiménez et al. (1999) was used, consisting of two glass vessels, one serving as culture vessel in which the explants were placed and the other as culture medium reservoir.
In the first phase each system contained 2 000 ml multiplication medium. TIS were incubated under cool white fluorescent tubes (125-150 μmol m-2s-1, 16 h photoperiod) for shoot growth and temporarily immersed 2 minutes every 3 hours for shoot growth. After 21 days the whole culture medium was exchanged by a volume of 3 500 ml culture medium for tuber induction and plants were incubated in darkness for six weeks and temporarily immersed every 6 hours.
Each experiment had four replicates and was repeated twice.
Inoculum density
Two inoculum densities were tested: 60 and 90 single nodal segments were inoculated per TIS vessel. Immersion times during the tuberization stage were 2 minutes every 6 hours.
Immersion times during tuberization stage
In the tuberization stage Temporary Immersion Systems inoculated with 60 single nodal segments were temporarily immersed during 2 and 30 minutes every 6 hours.
Field studies
Direct field planting of potato microtubers produced in TIS was compared with plants propagated by in vitro cuttings and transplanted to the field.
Prior to transplanting, microtubers with a diameter exceeding 4.0 mm were immersed in a solution of Giberelic Acid (0.05 g.l-1) for five minutes and stored at room temperature in a ventilated growth cabine under continuous light of small incandescent bulbs (6V AC / 0.05 A, 8 pro square meter, 25-40 cm above the tubers). After 21 days of storage microtubers were planted directly into the field in a red ferralitic soil.
A randomized block design with four replicates was used for field trials. The experimental plots were formed by four furrows with a total of 120 microtubers or in vitro plants per replicate planted at a distance of 0.90 x 0.20 m (Agramonte, 1999).
Data recording and statistical analysis
Number and total weight of the microtubers per TIS, the number of microtubers per plant, as well as the average fresh weight (g) and the diameter (mm) of the tubers at the end of the tuberization stage (six weeks) were recorded and microtubers classified into three categories: < 4.0 mm, 4.0 - 7.0 mm and > 7.0 mm diameter. Occurrence of deformed microtubers was also evaluated.
In field experiments, percentage of surviving plants was evaluated 15 and 35 days after planting. Height (cm) and stem number per plant were determined 45 days after planting. Tubers were harvested manually 70 days after planting and the number of minitubers per plant, fresh weight per plant (g) and minituber diameter were evaluated in 60 plants per replicate located in the internal furrows of each plot, for a total of 240 plants evaluated per treatment.
Data were processed using ONEWAY analysis of variance, after testing homogeneity of variances. The statistical computacional package used was SPSS/PC version 9.0 for Windows.
For the analysis of the percentage parameters classifying the microtubers according to caliber the Test of Hypothesis for binomial proportions was applied using the Statistical Package Statgraphics version 2.1.
RESULTS AND DISCUSSION
Effect of inoculum density on microtuber production and quality
The inoculum densities studied did not affect the normal development of the shoots during shoot growth stage. Plant morphology was similar in both treatments; no changes in color and vigor of the plants were observed.
During the tuberization stage, in the treatment with 90 explants per TIS the shoots showed excessive enlargement and strangulation in the apical area; calli with watery consistency were formed on the surface of shoots. The symptoms described are probably due to low oxygen availability inside the culture vessel because of the high amount of biomass production.
Microtubers formation was observed in both treatments after seven days into the tuberization stage. The average number of microtubers obtained per TIS was 168 in the treatment with 60 explants, and significantly higher with 234 in the treatment with 90 explants. However, the total fresh weight of microtubers per TIS was 164.7g in the treatment with 60 explants while with 90 explants only 47 g fresh weight was reached.
Further, an average of 23.1% deformed tubers was observed in the treatment with 90 explants, with the remaining the microtubers characterized by a watery consistency and blackened base; one week after harvest complete dehydration had occurred. Microtubers obtained in the treatment with 60 explants were of increased weight and size (Table 1) and showed none of the symptoms described.
Sarkar et al. (1997) and Piao et al. (2003) have reported the necessity to optimize the number of plants per culture vessel in order to ensure a rapid growth in potato micropropagation. In TIS, due to the higher volume of the vessels compared to the flasks used in conventional micropropagation, the use of small inoculum densities could cause the sub-utilization of the culture vessels and the capacities of the culture rooms (unpublished data). On the other hand, high densities may cause phenotypic malformations and decrease the quality of the plants or microtubers, as demonstrated in this experiment. Therefore, it is very important to evaluate this factor experimentally to take maximum advantage of the capacity of the vessels without affecting microtuber quality adversely.
Effect of immersion time during the tuberization stage on microtuber production and quality
In the treatment with two minutes of immersion time microtuber formation was observed six days after initiating conditions for tuber induction, while in the treatment with 30 minute immersions tuberization commenced six days later.
TIS with two minutes immersion time produced more microtubers (187), with increased fresh weight (164.9 g) and size compared to TIS with 30 minutes immersion time (136 with 69.3 g of total fresh weight) (Table 2).
The higher microtuber formation with two minutes of immersion also resulted in an increased percentage of larger microtubers (>7.0 mm) as shown in figure 1, with better shape and more resistance to dehydration. Thirty minutes immersion time resulted in poor quality of the microtuberization process with callus formation around the lenticels, rough surfaces and sprouted buds.



Akita and Takayama (1994) and Teisson and Alvard (1999) used longer immersion times (one hour) for potato microtuber induction. Jiménez et al (1999) obtained the highest growth rates and tuber production with immersion times of 5 minutes. Immersion time is a fundamental factor to control nutrient assimilation as well as to minimize hyperhydricity in tissues cultivated in TIS. We demonstrated that for potato microtuber production in TIS, shorter immersion times (2 minutes) result in higher microtuber tuber numbers per plant, as well as larger size and better quality than longer immersion times.
Field studies
Fifteen days after field planting 89% of the microtubers sprouted and subsequently produced vigorous plants. Of the in vitro plants established in the field 77.3% had survived after 35 days. Agramonte (1999) obtained less than 50% of plants established from microtubers produced in semisolid medium (average size 4.0 mm) and Pruski et al. (2003a) only 40-63%.
Struik and Lommen (1999) reported that in vitro plants showed more vigor than plants from microtubers obtained in semisolid medium. However, in the present field study plants grown from TIS produced microtubers reached an average height of 22.7cm and 1.5 stems per plant compared to an average height of 15.3 cm and 1.16 stems per plant reached by transplanted in vitro plants.
Pruski et al. (2003b) found that most of the plants derived from in vitro plantlets produced excessive foliage with extensive branching of the lower stems compared to plants grown from microtubers obtained in semisolid medium. The plants derived from TIS produced did not show excessive branching.
Agramonte (1999) obtained higher minituber production from transplanted in vitro plants compared to microtubers from semisolid medium, as did Pruski et al. (2003b) who reported that yield of seed tubers produced from microtubers were only 30 or 40% compared to in vitro plantlets. Pruski et al. (2003b) and Struik and Lommen (1999) observed better uniformity in minitubers from in vitro plants than microtuber derived plants. Since Kim et al. (1999) and Yu et al. (2000) reported that the size of the tubers destined for field plantation has an important effect on the performance of the resulting potato crop, microtubers from semisolid medium cannot be recommended for the production of minituber seed in the field.
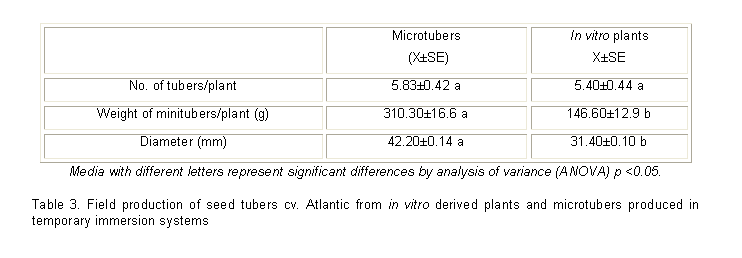
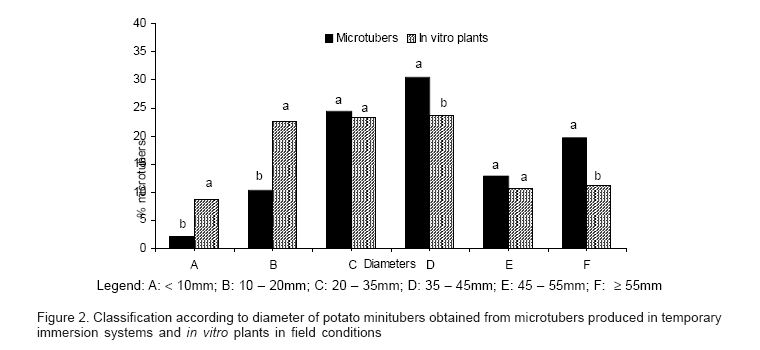
In the present study using TIS derived microtubers, the number of minitubers harvested per plant was similar to in vitro plants established in the field, while fresh weight and diameter of minitubers was higher in microtuber derived plants (Table 3). Plants derived from microtubers produced a higher number of tubers of class C (20 35mm), D (35 45mm), E (45 55mm) and F (> 55mm) than plants from in vitro plants (figure 2).
CONCLUSIONS
Microtubers are suitable for semiautomatic propagation systems and easier to handle than in vitro plants. A greenhouse acclimatization stage can be dispensed with, increasing the efficiency of the seed production process.
Temporary Immersion Systems (TIS) have proved advantageous for microtuber production compared to propagation strategies using semisolid culture media. Culture parameters such as inoculum density and immersion time must be optimized to ensure the efficiency of microtuber production and quality in TIS. In the present study the best results were obtained with 60 explants per TIS and two minutes immersion time during tuberization.
ACKNOWLEDGEMENTS
The authors wish to thank the support of the Cuban Ministry of Science, Technology and Environment (CITMA).
REFERENCES
Agramonte D (1999) Métodos biotecnológicos para la producción de semilla original de papa (Solanum tuberosum L.). Doctoral Thesis. Universidad Central de Las Villas, Instituto
Akita M & Ohta Y (1998) A simple method for mass propagation of potato (Solanum tuberosum L.) using a bioreactor without forced aeration. Plat Cell Report 18: 284-287
Akita M & Takayama S (1994) Induction and development of potato tubers in a jar fermentor. Plant Cell, Tiss Org Cult 36: 177-182
Etienne H & Berthouly M (2002) Temporary immersion systems in plant micropropagation. Plant Cell, Tiss Org Cult 69: 215231
Coleman WK, Donnelley DJ & Coleman SE (2001) Potato microtubers as research tools: a review. Am J Potato Res 78: 47-55
Duplessis T, Lewis T, Maskewich L & Andrew K (2000) Seed Potato Program [online]: Crop Diversification Centers 1999 Annual Report. http://www.agric.gov.ab.ca/ministry/pid/cdc/ seedpotato_ar.html
Jiménez E, Pérez N, de Feria M, Barbón R, Capote A, Chávez M, Quiala E & Pérez JC (1999) Improved production of potato (Solanum tuberosum L.) microtubers using a temporary immersion system. Plant Cell, Tiss Organ Cult 59: 19-23
Kim SY, Kim JK, Choi KH, Joung YH & Joung H (1999) Effects of Rindite on breaking dormancy of potato microtubers. Am J Potato Res 76: 5-8
Lê CL (1999) In vitro microtuberization: an evaluation of culture conditions for production of virus-free potatoes. Potato Res 42: 489-498
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473-479
Piao XCh, Chakrabarty D, Hahn EJ & Paek KY (2003) A simple method for mass production of potato microtubers using a bioreactor system. Current Science 84 (8): 1129-1132
Pruski K, Astatkie T, Duplessis P, Lewis T, Nowak J & Struik PC (2003a) Use of Jasmonate for conditioning of potato plantlets and microtubers in greenhouse production of minitubers. Amer J of Potato Res 80: 183-193
Pruski K, Astatkie T, Duplessis P, Stewart L, Nowak J & Struik PC (2003b) Manipulation of microtubers for direct field utilization in seed production. Amer J of Potato Res 80: 173-181
Pruski K, Astatkie T & Nowak J (2002) Jasmonate effects on in vitro tuberization and tuber bulking in two potato cultivars (Solanum tuberosum L.) under different media and photoperiod conditions. In vitro Cell Dev Biol-Plant 38: 203-209
Sarkar D, Chandra R & Naik P (1997) Effect of inoculation density on potato micropropagation. Plant Cell, Tiss Org Cult 48: 63-66
Struik P & Lommen W (1999) Improving the field performance of micro- minitubers. Potato Res 42: 559-568
Struik PC & Wiersema SG (1999) Seed potato technology. The Netherlands: Wageningen pers: 383
Teisson C & Alvard D (1999) In vitro production of potato microtubers in liquid medium using temporary immersion. Potato research 42: 499-504
Yu W, Joyce P, Cameron D & McCown B (2000) Sucrose utilization during potato microtuber growth in bioreactors. Plant Cell Reports 19: 407-413
Ziv M, Ronen G & Raviv M (1998) Proliferation of meristematic clusters in disponsable presterilized plastic bioreactors for the large-scale micropropagation of plants. In vitro Cell Dev Biol.-Plant 34: 152-158
Copyright (c) 2016 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
