ARTÍCULO ORIGINAL
Biotecnología
Vegetal Vol. 15, No. 3: 151 - 156, julio - septiembre, 2015
ISSN 2074-8647, RNPS: 2154 (Versión electrónica)
Instituto de Biotecnología de las Plantas. UCLV. MES.
The effects of plant growth promoting Bacillus pumilus
CCIBP-C5 on `Grande naine' (Musa AAA) plants in acclimatization
stage
Efecto de bacteria promotora del crecimiento vegetal Bacillus pumilus CCIBP-C5 sobre plantas de `Grande naine' (Musa AAA) en fase de aclimatización
Mileidy Cruz-Martín*, Eilyn Mena, Cynthia Sánchez-García, Berkis Roque, Mayra Acosta-Suárez, Tatiana Pichardo, Michel Leiva-Mora, Yelenys Alvarado-Capó. *Author for correspondence
Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5. Santa Clara. Villa Clara. Cuba. CP 54 830. e-mail: mileidy@ibp.co.cu
ABSTRACT
The search of effective and sustainable technological alternatives to replace chemical fertilization it is a challenge for scientists. Positive actions of microorganisms on plants growth are known. Considering these criteria, the objective was to determine the effect of Bacillus pumilus CCIBP-C5 on `Grande naine' (Musa AAA) plants in acclimatization stage. The ability to fix atmospheric nitrogen and indoleacetic acid production in vitro was determined. Furthermore, micropropagated Musa sp. plants were inoculated by immersion of roots in bacterial suspension. B. pumilus was able to grow in culture media without nitrogen, and produced 28.9 ìg ml-1 of indoleacetic acid. Besides, B. pumilus increased significantly the height and stem thickness, modified roots architecture and enhanced fresh and dry weight of plants. The bacteria strain CCIBP-C5 favorably influenced the growth of plants.
Key words: Indoleacetic acid, PGPB, growth promotion
RESUMEN
La búsqueda de alternativas tecnológicas eficaces y sostenibles para la sustitución de la fertilización química es un reto para los científicos. Se conoce de efectos positivos de microorganismos sobre el crecimiento vegetal. Teniendo en cuenta estos criterios, el objetivo fue determinar el efecto de Bacillus pumilus CCIBP-C5 sobre plantas de `Grande naine' (Musa AAA) en fase de aclimatización. Se determinó in vitro la capacidad de fijar el nitrógeno atmosférico y de producir ácido indol acético. Además, plantas de Musa sp. micropropagadas se inocularon por inmersión de las raíces en la suspensión bacteriana. B. pumilus fue capaz de crecer en medio de cultivo sin nitrógeno y produjo 28.9 ìg ml-1 de ácido indol acético. Además, la cepa B. pumilus aumentó significativamente la altura, el grosor del tallo, modificó la arquitectura de las raíces y aumentó la masa fresca y seca de las plantas. La cepa CCIBP-C5 influenció favorablemente el crecimiento de las plantas.
Palabras clave: ácido indol acético, PGPB, promoción de crecimiento
INTRODUCTION
One of the critical points in the bananas and plantains production is related to fertilization. Generally, require large amounts of nitrogen (N2) and potassium (K) followed by phosphorus (P), calcium (Ca) and magnesium (Mg) to maintain high yields (Robinson, 1996).
However, the increased
employment of chemical fertilizers is undesirable, because (1) production is
an energetically expensive process and most of the energy is provided by the
consumption of nonrenewable sources such
as fossil fuels and (2) a substantial pollution is caused by the production
and use of mineral nitrogen fertilizers (Mia et al., 2010). Consequently,
search effective and sustainable technological alternatives to replace chemical
fertilization it is a challenge for scientists.
The possibility to use microorganisms that live associated with plants is a promising alternative for a sustainable agriculture. Many researchers suggested that Plant Growth Promoting Bacteria (PGPB) are effective as a bioenhancer and biofertilizer for banana plants. For instant, Mia et al. (2005) found enhanced root formation
in bananas, when PGPB strain was inoculated in plants. Meanwhile, the effect of the combined inoculation of the arbuscular mycorrhiza Glomus manihotis and a rhizobacteria consortium of Bacillus spp. on micropropagated banana plantlets, during the acclimatization stage increased growth parameters like shoot length, leaf area, total fresh and aerial dry weight (Rodríguez-Romero et al., 2005). Also, PGPB inoculation in bananas showed a significant amount of nitrogen fixation (Mia et al., 2010).
Current agricultural use bacterial-based products as pesticides, fungicides and stimulators of plant growth (Pérez-García et al., 2011). Considering these criteria, the objective was to determine the effect of Bacillus pumilus CCIBP-C5 on `Grande naine' (Musa AAA) plants in acclimatization stage.
MATERIALS AND METHODS Plant material
In vitro plants of `Grande naine' (Musa AAA) were propagated by organogenesis according to the protocol described by Orellana (2004). After them, plants were placed in polythene bags with sterilized mixture of compost and zeolite (80:20). The plants were arranged in a greenhouse, according to a completely randomized design. Bacterial strains and culture conditions
Bacillus pumilus CCIBP-C5 belonging to the Microbial Culture Collection of Applied Microbiology Laboratory from Instituto de Biotecnología de Las Plantas was used. B. pumilus was isolated from banana phyllosphere. Beside, this strain have in vitro antifungal activity against Mycosphaerella fijiensis (Poveda et al., 2010).
Bacterial strain was grown in 250 ml flask containing 100 ml of Nutrient Broth (Fluka) medium at 30°C and 120 rpm shaking until exponential growth phase, OD600 = 0.1, equivalent to 109 colony-forming units (cfu) ml-1.
An aliquot of biological material was taken from pure culture to evaluate the nitrogen fixation, Indoleacetic acid (IAA) quantification and plant promotion assay.
Bacterial strains characterization
In order to determine the ability to fix atmospheric nitrogen, B. pumilus strain was streaked onto nitrogen-free Winogradsky medium (Krieg and Holt, 1984). Petri dishes was incubated at 28°C for 72 h. It was reported as positive result if the strain was able to grow in this culture medium. Nutrient Agar culture medium (Fluka) was used as a growth control.
IAA production was determined according to colorimetric method (Patten and Glick, 2002) as described below. The B. pumilus strain was grown in culture medium Tryptone Soya Broth (BioCen) at 28°C for 48 h in darkness. Cells were removed by centrifugation (10 000 g x 15 min) and 1.0 ml of the supernatant was added to 500 µl Salkowsky reagent. The mixture was incubated at 30°C and darkness for 30 min. Absorbance was measured at 530 nm (UV-visible spectrophotometer, Genesys 6, Thermo Electron Corporation, USA). The result was reported in µg ml-1 calculated from an IAA (Duchefa) standard curve (1.25, 2.50, 5.00, 10.00, 20.00, 40.00 µg ml-1).
Plant growth promotion
The plants were inoculated with bacterial suspension, by dipping roots for 20 min. Plants without bacteria treatment were used as control. The evaluation of the plants were performed weekly until 60 days after inoculation. The plant height (cm), stem thickness (cm), root length (cm), number of leaves and fresh and dry weight (g) of foliage and roots were determined. The dry weight was determined by using an oven at 70°C for 72 h. In each treatment, ten plants were used and the experiment was repeated twice.
Statistical analysis
As data did not meet the assumptions of normality and homogeneity of variances, it were submitted to non-parametric test (p<0.05) using a Statistic Package for Social Science (SPSS) v.20 for Windows.
RESULTS AND DISCUSSION
B. pumilus CCIBP-C5 was able to grow in culture medium without nitrogen. Also, B. pumilus produced 28.9 ìg ml-1 of indoleacetic acid in vitro condition.
The selection of bacterial strains with the ability of nitrogen fixation and IAA production, as CCIBP-C5, will contribute to develop of new biofertilizer products. Results of different research have been indicated that biological nitrogen fixation process accounts for 65% of the nitrogen utilized in agriculture, and to be important in future sustainable crop production systems (Matiru and Dakora, 2004). Nitrogen is required for cellular synthesis of enzymes, proteins, chlorophyll, DNA and RNA, and is therefore important in plant growth and production of food and feed (Hayat et al., 2010). The employ of N2 fixing and phosphate solubilized bacteria in agriculture could to reduce the chemical fertilization use (Mia et al. 2005).
Some authors have been previously indicated the favourable effects of IAA on plant growth. This hormone increases the rate of xylem and root development (lateral and adventitious), control processes of vegetative growth and affects photosynthesis (Tsavkelova et al., 2006). Besides, IAA synthesized by bacteria may be involved at different levels in plant-bacterial interactions. In particular, plant growth promotion and root nodulation (Glick, 2012).
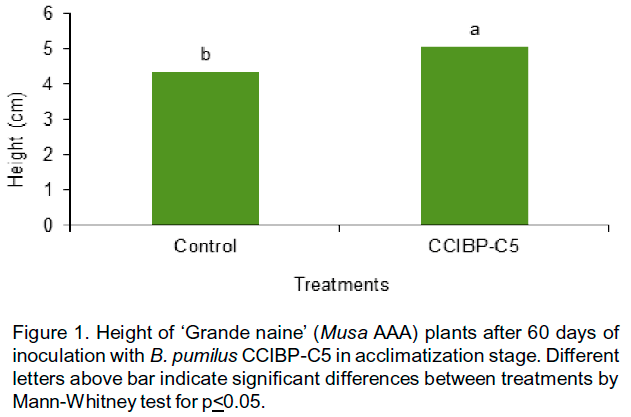
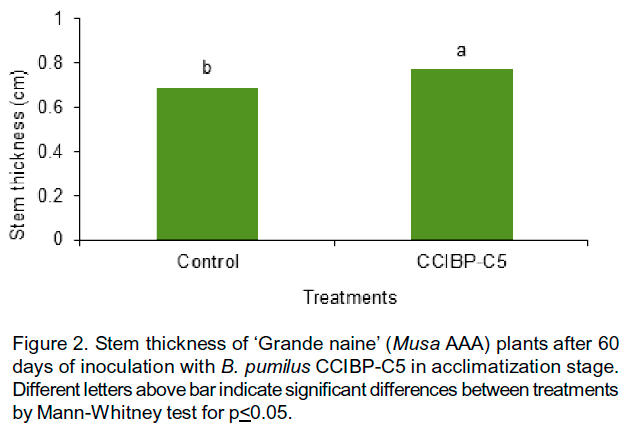
Plant growth promotion after the inoculation of B. pumilus CCIBP-C5 was observed. At 60 days after inoculation, CCIBP-C5 increased plant height (Figure 1) and stem thickness (Figure 2) respect to control.
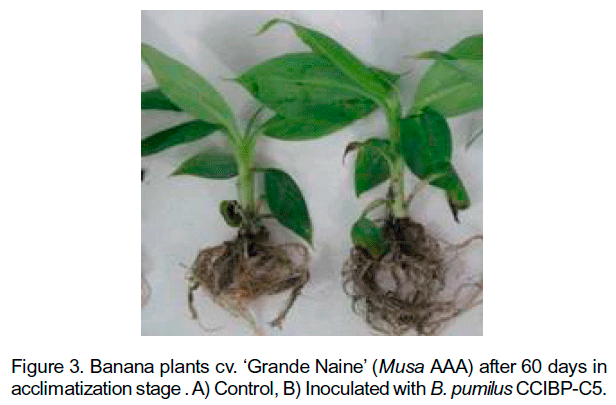
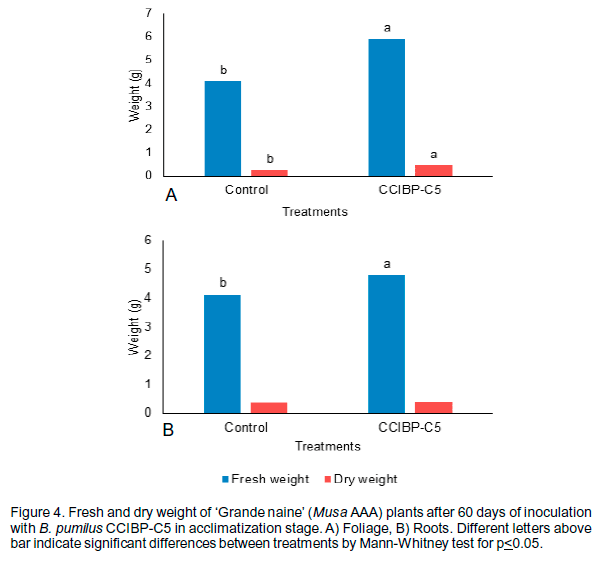
On the other hand, plants inoculated with B. pumilus CCIBP-C5 not showed differences in number of leaves per plant or root length respect to control with water. However, plants treated with the bacterial strain shows different radicle morphology, presence of secondary lateral roots (Figure 3) and significantly increase of the fresh and dry weight (Figure 4).
An increase in plant height has been observed by other authors like Galindo-Menéndez et al. (2009) using PGPB. Theirs found that a native strain of Azotobacter sp. Tu-24 stimulated the growth of Musa sp. cv. `FHIA-18' plants similar to treatment with BIOBRAS (commercial stimulators) and superior to control with water.
The result is in accordance with in vitro IAA production by CCIBP-C5 strain and previous reports. Is known that essential plant nutrients are taken up from the soil by roots. In this sense, good root growth is characteristic of auxin effect produced by bacteria and is one of the parameters used to determine the effectiveness of certain bacteria (Torres-Rubio et al., 2000). Many PGPR stimulates the root growth, sometimes via production of phytohormones by the plant or by the bacteria (Lucy et al., 2004). Promotion of root growth is considered a marker by which the beneficial effect of PGPB is measured. Overall, bacterial IAA increases root surface area and length, and thereby provides greater access to soil nutrients for plants (Glick, 2012).
According to the results of Rodríguez-Romero et al. (2005), banana plants treated with Bacillus sp. alone and in combination with Glomus manihotis had significantly increase in total fresh and aerial dry weight respect to control. A plant can grow vigorously if it contains much amount of fresh and dry weight (Mathivanan et al., 2014).
Bacteria have the potential to contribute in the development of sustainable agricultural systems (Mia et al., 2010). This contribution is by three different ways: synthesizing particular compounds such us auxin, facilitating the uptake of certain nutrients from the soil and lessening or preventing the plants diseases (Glick, 2012). Nitrogen fixation and plant growth promotion by plant growth promoting bacteria are important criteria for an effective biofertilizer.
In the case of Bacillus strains several reports indicated applications to enhance the growth of agricultural crops, wild plants, trees, microalgae, and model plants, through different mechanisms of plant growth-promotion (Kloepper et al., 2004; Bashan et al., 2010). In this work, B. pumilus CCIBP-C5 strain shows good promise as an inoculant for banana plant growth promotion in acclimatization stage. According to these results, the use of phyllosphere bacterial strains in sustainable agriculture could be an option to reduce the amounts of external inputs and time required for Musa spp. acclimatization.
ACKNOWLEDGEMENTS
This work was developed in the framework of institutional cooperation between the Central University Marta Abreu de Las Villas and the Council of Flemish Universities in Belgium (IUC UCLV / VLIR).
REFERENCES
Bashan Le, Hernandez JP, Bashan Y, Maier R (2010) Bacillus pumilus ES4: candidate plant growth-promoting bacterium to enhance establishment of plants in mine tailings. Environ Exp Bot 69(3): 343-352
Galindo-Menéndez L, Corría J, Arias M, Acosta E, Pérez N, Guntín P (2009) Efectos de cepas de Azotobacter chroococcum en plantas in vitro de banano (Musa sp. cv FHIA-18) en la fase de aclimatización. Revista de Innovación Tecnológica 15 (1): 168-176
Glick BR (2012) Plant growth promoting bacteria: Mechanisms and Applications. Scientifica: 10-15
Hayat R, Safdar A, Ummay A, Rabia K, Iftikhar A (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60: 579-598
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94: 1259 -1266
Krieg NR, Holt J (eds.) (1984) Bergey's Manual of Systematic Bacteriology. Williams & Wilkins. New York
Ladha JK, Reddy PM (1995) Extension of nitrogen fixation to rice: necessity and possibilities. Geo J 35: 363-372
Lucy M, Reed E, Glick BR (2004) Application of free living plant growth-promoting rhizobacteria. Antonie van Leeuwenhoek 86: 1-25
Mathivanan S, Chidambaram ALA, Sundramoorthy P, Baskaran L, Kalaikandhan R (2014) Effect of combined inoculations of plant growth promoting rhizobacteria (PGPR) on the growth and yield of groundnut (Arachis hypogaea L.). Int. J.Curr. Microbiol. App. Sci 3(8): 1010-1020
Matiru VN, Dakora FD (2004) Potential use of rhizobial bacteria as promoters of plant growth for increased yield in landraces of African cereal crops. Afr J Biotechnol 3(1): 1-7
Mia MA, Shamsuddin ZH, Zakaria W, Marziah M (2005) High yielding and quality banana production through plant growth promoting rhizobacterial inoculation. Fruits 60: 179-185
Mia MA, Shamsuddin ZH, Mahmood M (2010) Use of plant growth promoting bacteria in banana: a new insight for sustainable banana production. Int J Agric Biol 12: 459-467
Orellana P (1994) Tecnología para la micropropagación in vitro de clones de Musa spp. Tesis para aspirar por el grado científico de doctor en ciencias Agrícolas. UCLV. IBP. Santa Clara. 120p.
Patten Ch, Glick B (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68: 3795-3801
Pérez-García A, Romero D, Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol 22: 187-193
Poveda I, Cruz-Martín M, Sánchez-García C, Acosta-Suárez M, Leiva-Mora M, Roque B, Alvarado-Capó Y (2010) Caracterización de cepas bacterianas aisladas de la filosfera de Musa spp. con actividad antifúngica in vitro frente a Mycosphaerella fijiensis. Biotecnología vegetal 10(1): 57-61
Robinson JC (1996) Bananas and Plantains, pp.172-174. CAB International. Oxon
Rodríguez-Romero AS, Guerra MS, Jaizme-Vega MD (2005) Effect of arbuscular mycorrhizal fungi and rhizobacteria on banana growth and nutrition. Agron Sust Devel 25: 395-399
Torres-Rubio M, Valencia-Plata S, Bernal-Castillo J, Martínez-Nieto P (2000) Isolation of Enterobacteria, Azotobacter sp. and Pseudomonas sp. producers of indoleacetic acid and siderophores from Colombian rice rhizosphere. Revista Latinoamericana de Microbiología 42: 171-176
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI (2006) Microbial producers of plant growth stimulators and their practical use: a review. Applied Biochemistry and Microbiology 42(2): 117-126
Recibido: 21-07-2014
Aceptado:
18-12-2014
Copyright (c) 2016 Biotecnología Vegetal
![]()
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.