Artículo original
Biotecnología Vegetal Vol. 17, No. 3: 171 - 180, julio - septiembre, 2017
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Effect of inoculation time on Agrobacterium-mediated transformation efficiency of Musa cv. ‘Grande naine’ (AAA)
Efecto del tiempo de inoculación en la eficiencia de la transformación mediada por Agrobacterium de Musa cv. ‘Grande naine’ (AAA)
Mairenys Concepción-Hernández, Maritza Reyes, Mayelín Rodríguez, Rafael Gómez-Kosky, Borys Chong-Pérez
Instituto de Biotecnología de las Plantas, Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5,5. Santa Clara. Villa Clara. Cuba. CP 54 830. e-mail: mairenys@ibp.co.cu
ABSTRACT
Increasing inoculation time during Agrobacterium-mediated transformation has been shown to favour transformation efficiency in several plant species. The effect of inoculation time in combination with spermidine (Spd) on efficiency was determined during Agrobaterium-mediated transformation of the Musa cultivar ‘Grande naine’ (AAA). Banana embryogenic cell suspensions (ECSs) were incubated with the bacterial strain EHA 105 carrying the binary vector pFAJ3000. Six different inoculation conditions (6 h, 6 h+Spd, 12 h, 12 h+Spd, 24 h, 24 h+Spd) were compared based on transient GUS expression and number of embryo colonies formed. Moreover, leaf fragments from 24 randomly chosen regenerated plantlets were assayed presence and expression of the transgenes. Consequently, samples that were inoculated for 24 h in medium supplemented with 1 mM spermidine showed the highest transformation efficiency, expressed as number of blue foci and regenerated colonies after the selection treatment. Here we showed for the first time that longer inoculation times in combination with spermidine enhance the efficiency of Agrobacterium-mediated transformation of the banana cultivar ‘Grande naine’.
Keywords: banana, β-glucuronidase, genotype, somatic embryo, PCR
RESUMEN
El incremento en el tiempo de inoculación durante la transformación mediada por Agrobacterium ha mostrado que aumenta la eficiencia de la transformación en varias especies de plantas. En este trabajo se determinó el efecto del tiempo de inoculación en combinación con la adición de espermidina (Spd) en la eficiencia de la transformación genética mediada por Agrobacterium tumefaciens del cv. ‘Grande naine’ (Musa AAA). Las suspensiones celulares embriogénicas de banano fueron inoculadas con la cepa bacteriana EHA 105 que contiene el vector binario pFAJ3000. Se compararon seis condiciones de inoculación (6 h, 6 h+Spd, 12 h, 12 h+Spd, 24 h, 24 h+Spd) en cuanto a la expresión transitoria GUS y el número de colonias embriogénicas formadas. Además, se analizaron fragmentos de hojas de 24 plántulas regeneradas para la presencia y expresión de los transgenes. Consecuentemente, las muestras por 24 h con 1 mM de espermidina mostraron la mayor eficiencia de transformación, expresada en número de puntos azules y colonias regeneradas después de la selección. En este trabajo se muestra por primera vez que el aumento del tiempo de inoculación en combinación con el uso de espermidina incrementa la eficiencia de la transformación genética mediada por Agrobacterium en el cultivar ‘Grande naine’ de banano.
Palabras clave: banano, β-glucuronidasa, genotipo, suspensiones celulares embriogénicas, PCR
INTRODUCTION
Bananas and plantains (Musa spp.) are staple food for over 400 million people worldwide, serving as a rich source of carbohydrates, vitamins and mineral elements like potassium, phosphorous, calcium and magnesium (Mohapatra et al., 2010).
The banana cv. ʽGrande naineʼ (Musa AAA) is one of the most commercialized cultivar worldwide due to its resistance to wind throw and production of large bunches and fingers despite its relatively small stature (Ploetz et al., 2007). This cultivar shows enhanced resistance against races 1, 2 and 3 of the devastating pathogen Fusarium oxysporum L. f. sp. cubense (Foc), whereas it is highly susceptible to the Black Leaf Streak Disease caused by Mycosphaerella fijiensis Morelet and the emerging tropical race 4 of Foc (Robinson and Galán, 2010). Due to polyploidy, long life cycle and high sterility found in this and most of edible cultivars, genetic transformation arises as an attractive means for introducing agronomically important traits and performing functional genomics analysis in Musa species (Roux et al., 2008). Therefore, the development of protocols that render high transformation efficiencies and could be used for several cultivars is essential (Khanna et al., 2004).
Since Sági et al. (1994) first reported the genetic transformation in this genus through protoplast electroporation, several methods have been developed. The Agrobacterium tumefaciens-mediated transformation of ECSs is the most commonly used (Ganapathi et al., 2001; Khanna et al., 2004; Pérez-Hernández et al., 2006 a; Ghosh et al., 2009; Chong-Pérez et al., 2012 a). Likewise, several parameters have been modified in order to increase the transformation efficiency. Recently, Chong-Pérez et al. (2012 a) reported the use of spermidine (Spd) and modifications on inoculation time (30 min and 6 h) in a centrifugation-assisted transformation protocol to increase transformation efficiency of banana cv. ‘Dwarf Cavendish’ (Musa AAA), with the best results for 6 h of inoculation in the presence of spermidine.
Increasing inoculation time during Agrobacterium-mediated transformation has been shown to favour transformation efficiency in several plant species (Dan and Ow, 2011). However, the use of longer inoculation times is limited by the induction of defense responses in the plant tissues, which typically involve necrosis or programmed cell death (Hansen, 2000; Zhang et al., 2013). Thus, the regeneration of explants and consequently the transformation efficiency are compromised. Spermidine is a major polyamine in plants that has been used in genetic transformation since it favours embryo multiplication and regeneration (Petri et al., 2005; Silva et al., 2009) and enhance T-DNA transfer and activation of vir genes when added exogenously (Kumar and Rajam, 2005).
Since genetic transformation in Musa species is far for being a stablished routine, mostly due to transformation dependency on genotype, the main objective of this work was to determine whether increasing the inoculation time of embryogenic cell aggregates with A. tumefaciens in the presence of spermidine influence transformation efficiency in Musa cv. ʽGrande naineʼ.
MATERIALS AND METHODS
Inoculum preparation
The A. tumefaciens strain EHA-105 carrying the binary vector pFAJ3000 (De Bondt et al., 1994) was used in this study (Figure 1). The bacteria were grown on semi-solid LB medium supplemented with spectinomycin 100 mg l-1, streptomycin 300 mg l-1 and rifampicin 50 mg l-1, and incubated at 28 °C for 24–48 h. Resulting single colonies were cultured in 3 ml selective liquid LB medium and incubated at 200 rpm and 28±2 °C for 16–24 h to an A600 of approximately 1.2 units (Biophotometer, Eppendorf, Germany). Then, 50 ml selective liquid YEP medium were inoculated with 50 µl of this culture and kept under the preceding conditions to an A600 of 1.2 units. After that, the culture was centrifuged for 10 min at 3 430 x g (Eppendorf, Germany) and the cells were re-suspended in liquid ZZ medium (half strength Murashige and Skoog (MS) medium (Murashige and Skoog, 1962), MS vitamins, 10 mg l-1 ascorbic acid, 30 g l-1 sucrose, 1 mg l-1 2,4-D, pH 6.12 before autoclaving) supplemented with 200 µM acetosyringone. The A600 was adjusted between 0.4 and 0.5 units (~109 cfu ml-1).

Plant material
Embryogenic cell suspensions (ECSs) from Musa cv. ‘Grande naine’ (AAA) were obtained from immature male flowers as described by Côte et al. (1996).
Genetic transformation and plant regeneration
Genetic transformation of cell aggregates and plant regeneration were performed according to Chong-Pérez et al. (2012 b). Two hundred microliter of 33% settled cell volume (SCV) ECSs in ZZ liquid medium were mixed with one milliliter of bacterial suspension in 24-well plates and incubated at 25°C and 25 rpm in the dark. During inoculation with the bacteria, five different conditions (6 h, 12 h, 12 h+Spd, 24 h, 24 h+Spd) were compared to the standard condition(6 h with 1 mM Spd) (Chong-Pérez et al., 2012 a). After that, cells were transferred to a nylon mesh, placed in Petri dishes containing ZZ medium, 3 g l-1 Gelrite and 200 µM acetosyringone, pH 6.3 before autoclaving (final pH after autoclaving ~5.6), and kept in the dark at 21°C for six days. Then, cell aggregates were shifted to semi-solid ZZ medium, pH 6.12 before autoclaving, containing 200 mg l-1 timentin (to eliminate the bacteria) and 50 mg l-1 geneticin (to select for transformed cells), and kept in the dark at 27 ± 2°C for eight weeks, with bi-weekly subcultures. Next, the obtained embryo colonies (groups of proembryos and embryos derived from a single embryo, and consequently from the same transformation event) were placed in antibiotic containing RD1 medium (half strength MS medium, MS vitamins, 100 mg l-1 ascorbic acid, 100 mg l-1 myo-inositol, 2.5 g l-1 Gelrite, 30 g l-1 sucrose, pH 5.8) for four weeks in the dark at 27 ± 2°C. Resulting embryos were transferred to maturation medium (MS medium, MS vitamins, 0.25 mg l-1 6-bencylaminopurine (6-BAP), 0.75 mg l-1 indole-3-acetic acid (IAA), 100 mg l-1 myo-inositol, 45 g l-1 sucrose, 2.5 g l-1 Gelrite, pH 5.8) for another three weeks. Afterwards, mature embryos were placed in germination medium (MS medium, 1.0 mg l-1 biotin, 0.5 mg l-1 6-BAP, 2.0 mg l-1 IAA, 45 g l-1 sucrose, 2.5 g l-1 Gelrite, pH 5.8) at 27 ± 2°C under solar light conditions. After four weeks in this conditions, regenerated plants were transferred to elongation medium (MS medium, 30 g l-1 sucrose, 2.5 g l-1 Gelrite, pH 5.8) for another month.
Histochemical β-glucuronidase (GUS) assays
Histochemical GUS assay was conducted as described before (Jefferson et al., 1987; Chong-Pérez et al., 2012 a) on embryogenic cell aggregates and leaf segments. Transient GUS expression (TGE), expressed as the average number of blue foci of 30 samples per treatment (200 µl at 33% settled cell volume of ECSs ~50 mg fresh weight) was determined. In order to determine genetic transformation efficiency, the number of blue foci on embryogenic cells and embryo colonies were determined after the co-culture and the eight weeks selection period, respectively. Likewise, 1 cm2 leaf segments sliced from 24 randomly chosen regenerated plantlets (four plantlets per treatment) were incubated in the assay solution supplemented with 0.2% SDS and 1% (w/v) ascorbic acid and subjected to 66.6 kPa vacuum for 10 min to favor substrate penetration into the tissues. Incubation was performed as above and the tissues were washed in 70% (v/v) ethanol to improve color contrast. Consequently, β-glucuronidase stable expression was qualitatively assessed through the presence of blue zones.
DNA isolation and PCR analysis
The above 24 plantlets were also analysed by PCR. The genomic DNA was isolated according to the protocol of Khayat et al. (2004) with modifications described by Chong-Pérez et al. (2012 b). The sequence of the primers used to detect the presence of the uidA-intron and nptII genes were Fw 5’-TGGGCAGGCCAGCGTATCGT-3’, Rv5’-ATCACGCAGTTCAACGCTGAC-3’, and Fw 5´-ATGATTGAACAAGATGGATTGCACGC-3´, Rv 5´-TGATGCTCTTCGTCCAGATCATC-3´, respectively. The expected fragments from the amplification reactions were 609 bp and 488 bp length, respectively. The reaction was performed in 25 µl final volume mixture containing 0.4 μg of genomic DNA, 0.5 μM of each primer, 200 μM dNTPs, 1X DreamTaq buffer, 1 unit DreamTaq polymerase (Fermentas, Germany), 1% BSA and 1% (w/v) PVP. Thermocycling was carried out in a Mastercycler programmable thermal control (Eppendorf, Germany) and started with denaturation at 94 °C for 4 min, followed by 30 cycles at 94 °C for 45 s, 66 °C for 45 s and 72 °C for 45 s, with a final extension at 72 °C for 10 min. The amplified fragments were analysed by 1.5% (w/v) agarose gel electrophoresis performed at 10 V/cm in 1X Tris-borate-EDTA buffer and stained with 0.5 µg ml-1 ethidium bromide.
Statistical analysis
Data were processed using the IBM Statistical Package for Social Sciences (SPSS) for Windows Version 22.0 and non-parametric tests H of Kruskal Wallis and U of Mann Whitney were used with a significance of p<0.05.
RESULTS AND DISCUSSION
In this work, the effect of inoculation time and spermidine in transformation efficiency was determined by measuring TGE in cell aggregates and the number of embryo colonies after the selection procedure. For the first variable, no significant differences were observed among samples treated and non-treated with spermidine, while a significant increase in TGE was provoked by inoculation times longer than 6 h, without differences between the 12 h and 24 h treatments (Table 1, Figure 2). A similar effect on TGE for this cultivar was previously reported by Arinaitwe et al. (2004) when increasing inoculation times from 4 h to 14 h. Apparently, longer inoculation times may favour the initial steps of chemotaxis, bacteria attachment and activation of vir genes during Agrobacterium-mediated transformation and increase TGE.
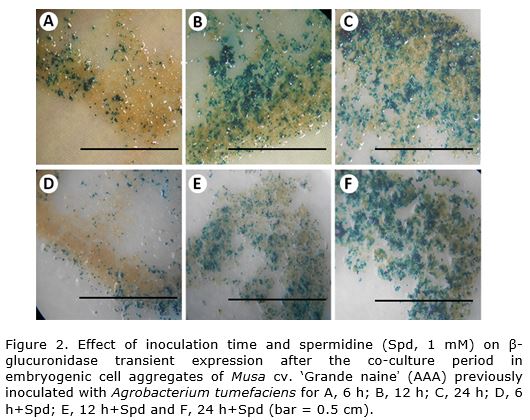
At the end of the two-month selection period, small embryo colonies were visible in the surface of the cell aggregates, while no embryo formation was detectable in untransformed controls placed on selective medium.

The spermidine-treated samples showed higher stable transformation frequencies when inoculation times were increased up to 12 and 24 h (Table 1), being the highest number of embryo colonies obtained with the 24 h+Spd treatment. The former treatment also showed no significant differences with untransformed controls. Furthermore, no differences among each other were found on the treatments without spermidine, while samples with and without the polyamine differed only when the inoculation times were increased. This suggests an additive effect of both, inoculation time and spermidine.
A similar performance was reported by Khanna et al. (2004) during a heat shock treatment of 45°C for 5 min to ‘Grande naine’ and ‘Lady Finger’ (Musa AAB) ECSs before inoculation with Agrobacterium. The treatment did not increase the transient GFP expression, while it doubled the formation of embryo colonies. Moreover, Chong-Pérez et al. (2012 a) reported that 1 mM spermidine increased the number of embryo colonies and regenerated plants of cv. ‘Dwarf Cavendish’ (Musa AAA), as well as the TGE in embryogenic cell aggregates with an inoculation time of 6 h. The latter indicates that plant genotype might influence this response. Seemingly, spermidine has a protective effect on banana cell aggregates during Agrobacterium infection, which became more patent when increasing the inoculation time.
Is known that as a plant pathogen, A. tumefaciens elicits defense responses in plant tissues. Veena et al. (2003) described the transcriptional elicitation of defense genes in tobacco BY-2 cell cultures after inoculation with several A. tumefaciens strains. Also, in Arabidopsis thaliana, defense responses induced by the pathogen-associated molecular pattern EF-Tu reduce transformation by A. tumefaciens (Zipfel et al., 2006). A similar induction of defense genes have been reported for Arabidopsis cell cultures (Ditt et al., 2006) and inflorescence stalks (Lee et al., 2009), and wheat embryogenic calluses (Zhou et al., 2013) inoculated with A. tumefaciens. Among these responses, the occurrence of programmed cell death (PCD) is a major factor that negatively affects efficiency during Agrobacterium-mediated transformation of several plant species (Hansen 2000; Zhang et al., 2013). PCD can be triggered as a result of the oxidative burst in plant cells following interaction with the bacteria, where H2O2 acts as a signal and regulator (Kaurilind et al., 2015). Thus, a recent study showed that Agrobacterium-mediated H2O2 accumulation led to cell death during genetic transformation of tomato (Solanum lycopersicum L.) cotyledons (Dan et al., 2016). Also, the antioxidant defense systems and anti-PCD signaling cascades are inhibited during H2O2-induced PCD in tobacco cell suspensions (Vannini et al., 2012). Banana ECSs undergo apoptotic-like PCD after inoculation with Agrobacterium (Khanna et al., 2007), although whether cell death is triggered by H2O2 accumulation is still unknown.
Polyamines are related with PCD regulation (Moschou and Roubelakis-Angelakis, 2014) and spermidine, putrescine and spermine have been reported to modulate stress-triggered ROS homeostasis and oxidative damage (Moschou and Roubelakis-Angelakis, 2014; Sudhakar et al., 2015). There is growing evidence that polyamine accumulation is associated with induction of antioxidant enzymes like superoxide dismutase, catalase and ascorbate peroxidase, consequently reducing free radicals levels and oxidative damage (Liu et al., 2015; Sudhakar et al., 2015). For instance, the exogenous addition of spermidine in tobacco (Nicotiana tabacum) cell suspensions protected the cells from heat-shock-induced PCD and rebounded the ascorbate peroxidase suppression (Marsoni et al., 2010). Similar results were obtained by this group when H2O2-induced PCD in tobacco cells was delayed by exogenous addition of spermidine (Vannini et al., 2012). This might explain the protective effect of spermidine observed in this study. Nevertheless, further studies are needed to elucidate the role of this polyamine during Agrobacterium-mediated transformation.
Additionally, of the 24 leaf fragments analysed for stable expression of the uidA gene, a total of 16 lines (66.6%) were positive for the GUS staining while the rest showed no coloration. The absence of GUS activity in these lines might be caused by several reasons: (i) the plants are not transgenic, (ii) the T-DNA is truncated or (iii) the uidA gene is silenced. In order to verify the previous conjectures, the presence of the genes nptII and uidA was analysed through PCR (Figure 3).

Fifteen lines were positive for the nptII and uidA fragments amplification, as well as for the GUS expression in leaves. However, lines 2, 3 and 4 (12.5%) were PCR negative, being escapes of the selective process. In addition, lines 1 and 16 were PCR positive for both genes but showed no GUS activity. This is probably due to the insertion of the T-DNA in a transcriptionally inactive region of the chromatin, in which case it should be considered as an escape. Also, the insertion of multiple copies of the transgenes might have negatively influenced the GUS expression (Rajeevkumar et al., 2015). Similarly, a deletion near the right border that affects expression but not the amplification might be a possible explanation for this phenomenon. Furthermore, line 7 was positive for uidA amplification and GUS activity but resulted negative for nptII. On the contrary, lines 8 and 15 were nptII positive and uidA and GUS negative, probably as a result of the loss of the gene during the T-DNA transfer and integration processes (Ziemienowicz et al., 2008). Few years ago there was a consensus that T-DNA was preferentially inserted in transcriptionally active region of the plant genome (Chen et al., 2003), though this statement was based on the analysis of transgenic plants subjected to a selective process. Nevertheless, Kim et al. (2007) proved that T-DNA integration in the genome of Arabidopsis plants that have not been submitted to selection was a random process that included centromeric and telomeric regions. In the current study, three lines with putative deletions on the right border and one in the left border of the T-DNA were identified. This results were independent of the inoculation treatment used (data not shown) and suggest the occurrence of modifications on both borders of the insert in Musa sp., which is consistent with what was previously reported for this species by Pérez-Hernández et al. (2006 b).
CONCLUSIONS
This work shows that longer inoculation times in combination with spermidine can be used to enhance Agrobacterium-mediated transformation of the relevant banana cultivar ‘Grande naine’. Also, it could have value for performing functional genomics analysis in Musa species. Finally, spermidine seems to protect the banana cells during infection with the bacteria.
ACKNOWLEDGEMENTS
The authors thank Novisel Veitía Rodríguez for assistance with statistical analysis.
REFERENCES
Arinaitwe G (2008) An improved Agrobacterium-mediated transformation method for banana and plantain (Musa spp.). Doctoral thesis dissertation, Catholic University Leuven, Leuven, Belgium
Arinaitwe G, Rem S, Strosse H, Swennen R and Sági L (2004) Agrobacterium- and particle bombardment-mediated transformation of a wide range of banana cultivars. In: Jain SM, Swennen R (eds). Banana improvement: Cellular, molecular biology, and induced mutations, pp. 99–109. Science Publishers, Plymouth
Chen S, Jin W, Wang M, Zhang F, Zhou J, Jia Q, Ping Wu (2003) Distribution and characterization of over 1000 T-DNA tags in rice genome. The Plant Journal 36(1): 105–113; doi:10.1046/j.1365-313X.2003.01860.x.
Chong-Pérez B, Reyes M, Rojas L, Ocaña B, Pérez B, Kosky RG, Angenon G (2012 a) Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in banana cv. ‘Dwarf Cavendish’ (Musa AAA): effect of spermidine on transformation efficiency. Plant Cell Tissue and Organ Culture 111(1): 79–109; doi:10.1007/s11240-012-0174-1
Chong-Pérez B, Kosky RG, Reyes M, Rojas L, Ocaña B, Tejeda M, Pérez B, Angenon G (2012 b) Heat shock induced excision of selectable marker genes in transgenic banana by the Cre-lox site-specific recombination system. Journal of Biotechnology 159(4): 265–273; doi:10.1016/j.jbiotec.2011.07.031
Côte F, Domergue R, Monmarson S, Schwendiman J, Teisson C, Escalant JV (1996) Embryogenic cell suspensions from the male flower of Musa AAA cv. ‘Grand naine’. Physiologia Plantarum 97(2): 285-290; doi:10.1034/j.1399-3054.1996.970211.x
Dan Y, Ow DW (2011) Plant transformation technology revolution in last three decades: historical technology developments in plant transformation. Bentham Science, Sharjah; ISBN: 1608052486
Dan Y, Zhang S, Matherly A (2016) Regulation of hydrogen peroxide accumulation and death of Agrobacterium-transformed cells in tomato transformation. Plant Cell Tissue and Organ Culture 127(1): 229-236; doi:10.1007/s11240-016-1045-y
De Bondt A, Eggermont K, Druart P, De Vil M, Goderis I, Vanderleyden J, Broekaert WF (1994) Agrobacterium-mediated transformation of apple (Malus x domestica Borkh.): an assessment of factors affecting gene transfer efficiency during early transformation steps. Plant Cell Reports 13(10): 587–593; doi:10.1007/BF00234517
Ditt RF, Kerr KF, de Figueiredo P, Delrow J, Comai L, Nester EW (2006) The Arabidopsis thaliana transcriptome in response to Agrobacterium tumefaciens. Molecular Plant-Microbe Interactions 19 (6): 665–681; doi:10.1094/MPMI-19-0665
Ganapathi TR, Higgs NS, Balint-Kurti PJ, Van Eck J (2001) Agrobacterium-mediated transformation of embryogenic cell suspensions of banana cultivar Rasthali (AAB). Plant Cell Reports 20 (2): 157–62; doi:10.1007/s002990000287
Ghosh A, Ganapathi TR, Nath P, Bapat VA (2009) Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in an important Cavendish banana cv. Robusta (AAA). Plant Cell Tissue and Organ Culture 97 (2): 131–139; doi:10.1007/s11240-009-9507-0
Hansen G (2000) Evidence for Agrobacterium-induced apoptosis in maize cells. Molecular Plant-Microbe Interactions 13 (6): 649–657; doi:10.1094/MPMI.2000.13.6.649
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6 (13): 3901–3907
Kaurilind E, Xu E, Brosché M (2015) A genetic framework for H2O2 induced cell death in Arabidopsis thaliana. BMC Genomics 16 (837); doi:10.1186/s12864-015-1964-8
Khanna H, Becker D, Kleidon J, Dale J (2004) Centrifugation assisted Agrobacterium tumefaciens-mediated transformation (CAAT) of embryogenic cell suspensions of banana (Musa spp. Cavendish AAA and Lady finger AAB). Molecular Breeding 14 (3): 239–252; doi:10.1023/B:MOLB.0000047771.34186.e8
Khanna HK, Paul JY, Harding RM, Dickman MB, Dale JL (2007) Inhibition of Agrobacterium-induced cell death by antiapoptotic gene expression leads to very high transformation efficiency of banana. Molecular Plant-Microbe Interactions 20 (9): 1048–1054; doi:10.1094/MPMI-20-9-1048
Khayat E, Duvdevani A, Lehav E, Ballesteros BA (2004) Somaclonal variation in banana (Musa acuminata cv. Grande Naine). Genetic mechanism, frequency, and application as a tool for clonal selection. In: Jain SM, Swennen R (eds). Banana improvement: Cellular, molecular biology, and induced mutations, pp. 99-109. Science Publishers, Plymouth; ISBN: 1-57808-340-0
Kim S, Veena, Gelvin SB (2007) Genome-wide analysis of Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. The Plant Journal 51 (5): 779–791; doi:10.1111/j.1365-313X.2007.03183.x
Kumar SV, Rajam MV (2005) Polyamines enhance Agrobacterium tumefaciens vir gene induction and T-DNA transfer. Plant Science 168 (2): 475-480; doi:10.1016/j.plantsci.2004.09.018
Lee CW, Efetova M, Engelmann JC, Kramell R, Wasternack C, Ludwig-Müller J, Deeken R (2009) Agrobacterium tumefaciens promotes tumour induction by modulating pathogen defense in Arabidopsis thaliana. Plant Cell 21 (9): 2948–2962; doi:10.1105/tpc.108.064576
Liu J-H, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: from synthesis to regulation. Frontiers in Plant Science 6 (827); doi:10.3389/fpls.2015.00827
Marsoni M, Cantara C, Pinto MC, Gadaleta C, Gara L, Bracale M, Vannini C (2010) Exploring the soluble proteome of Tobacco Bright Yellow-2 cells at the switch towards different cell fates in response to heat shocks. Plant Cell and Environment 33 (7): 1161–1175; doi:10.1111/j.1365-3040.2010.02137.x
Mohapatra D, Mishra S, Sutar N (2010) Banana and its by-product utilization: an overview. Journal of Scientific and Industrial Research 69: 323-329
Moschou PN, Roubelakis-Angelakis KA (2014) Polyamines and programmed cell death. Journal of Experimental Botany 65 (5): 1285-1296; doi:10.1093/jxb/ert373
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15 (3): 473-497; doi:10.1111/j.1399-3054.1962.tb08052.x
Pérez-Hernández JB, Remy S, Swennen R, Sági L (2006 a) Banana (Musa spp.). In: Wang K (ed). Methods in molecular biology vol 344: Agrobacterium protocols vol 2, pp. 167–175. Humana Press, Totowa; ISBN: 978-1-59745-131-4
Pérez-Hernández JB, Swennen R, Sági L (2006 b) Number and accuracy of T-DNA insertions in transgenic banana (Musa spp.) plants characterized by an improved anchored PCR technique. Transgenic Research 15 (2): 139–150; doi:10.1007/s11248-005-2544-5
Petri C, Alburquerque N, Pérez-Tornero O, Burgos L (2005) Auxin pulses and a synergistic interaction between polyamines and ethylene inhibitors improve adventitious regeneration from apricot leaves and Agrobacterium-mediated transformation of leaf tissues. Plant Cell Tissue and Organ Culture 82 (1): 105–111; doi:10.1007/s11240-004-7013-y
Ploetz CP, Kepler AK, Daniells J, Nelson SC (2007) Banana and plantain-An overview with emphasis on Pacific islands cultivars. In: Elevitch CR (ed). Species Profiles for Pacific Island Agroforestry, pp. 531-562. Permanent Agriculture Resources, Holualoa; ISBN: 0970254458
Rajeevkumar S, Anunanthini P, Sathishkumar R (2015) Epigenetic silencing in transgenic plants. Frontiers in Plant Science 6 (693); doi:10.3389/fpls.2015.00693
Robinson JC, Galán V (2010) Bananas and plantains. Crop production science in horticulture. CAB International, Wallingford; ISBN: 978-1-84593-658-7
Roux N, Baurens FC, Dolezel J, Hribova E, Heslop-Harrison P, Town C, Lagoda P (2008) Genomics of banana and plantain (Musa spp.), major staple crops in the tropics. In: Moore PH, Ming R (eds). Genomics of tropical crop plants, pp. 83–111. Springer, New York; ISBN: 978-0-387-71219-2
Sági L, Remy S, Panis B, Swennen R, Volckaert G (1994) Transient gene expression in electroporated banana (Musa spp. cv. Bluggoe, ABB group) protoplasts isolated from regenerable embryogenic cell suspensions. Plant Cell Reports 13 (5): 262–266; doi:10.1007/BF00233316
Silva TER, Cidade LC, Alvim FC, Cascardo JCM, Costa MGC (2009) Studies on genetic transformation of Theobroma cacao L.: evaluation of different polyamines and antibiotics on somatic embryogenesis and the efficiency of uidA gene transfer by Agrobacterium tumefaciens. Plant Cell Tissue and Organ Culture 99 (3): 287–298; doi:10.1007/s11240-009-9603-1
Sudhakar C, Veeranagamallaiah G, Nareshkumar A, Sudhakarbabu O, Sivakumar M, Pandurangaiah M, Lokesh U (2015) Polyamine metabolism influences antioxidant defense mechanism in foxtail millet (Setaria italica L.) cultivars with different salinity tolerance. Plant Cell Reports 34 (1): 141-156; doi:10.1007/s00299-014-1695-3
Vannini C, Marsoni M, Cantara C, Concetta De Pinto M, Locato V, De Gara L, Bracale M (2012) The soluble proteome of tobacco Bright Yellow-2 cells undergoing H2O2-induced programmed cell death. Journal of Experimental Botany 63(8): 3137–3155; doi:10.1093/jxb/ers031
Veena V, Jiang H, Doerge RW, Gelvin SB (2003) Transfer of T-DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defense gene expression. Plant Journal 35(2): 219–236; doi:10.1046/j.1365-313X.2003.01796.x
Zhang W, Dewey R, Boss W, Phillippy BQ, Qu R (2013) Enhanced Agrobacterium-mediated transformation efficiencies in monocot cells is associated with attenuated defense responses. Plant Molecular Biology 81(3): 273–286; doi:10.1007/s11103-012-9997-8
Zhou X, Wang K, Lv D, Wu C, Li J, Zhao P, Ye X (2013) Global analysis of differentially expressed genes and proteins in the wheat callus infected by Agrobacterium tumefaciens. PLoS One 8: e79390; doi:10.1371/journal.pone.0079390
Ziemienowicz A, Tzfira T, Hohn B (2008) Mechanisms of T-DNA integration. In: Tzfira T and Citovsky V (eds). Agrobacterium: from biology to biotechnology, pp. 395-440. Springer, New York; ISBN: 978-0-387-72289-4
Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125(4): 749–760; doi:10.1016/j.cell.2006.03.037
Recibido: 05-06-2017
Aceptado: 30-08-2017
Copyright (c) 2017 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
