Artículo original
Biotecnología Vegetal Vol. 17, No. 4: 221 - 227, octubre - diciembre, 2017
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Bacterial canker caused by Clavibacter michiganensis subsp. michiganensis in naranjilla in Ecuador
Cancro bacteriano asociado a Clavibacter michiganensis subsp. michiganensis en naranjilla en Ecuador
Carlos Bolanos-Carriel1, Patricio Gallegos1, José B. Ochoa1, María Insuasti1, Verónica Bonilla2, Mary H. Evans3, Jorge Alvarez4, Dario Ramirez4, Antonio León-Reyes4
1National Department of Plant Protection, Instituto Nacional de Investigaciones Agropecuarias-INIAP. km 1 Panamericana Sur. Quito. Ecuador. CP 17-01-340. e-mail: cbolanos@huskers.unl.edu
2National Department of Biotechnology, Instituto Nacional de Investigaciones Agropecuarias-INIAP. km 1 Panamericana Sur. Quito. Ecuador. CP 17-01-340.
3Ecuadorian Agency for Quality Assurance in Agriculture, AGROCALIDAD. Av Interoceánica km 14 ½. Tumbaco. Ecuador. CP 170184.
4Laboratorio de Biotecnología Agrícola y de Alimentos, Universidad San Francisco de Quito. Campus Cumbayá, Diego de Robles s/n. Quito. Ecuador. CP 17-1200-84.
ABSTRACT
Bacterial canker of naranjilla or lulo (Solanum quitoense and Solanum pectinatum) is a new disease that could completely destroy naranjilla plants. The objectives of this study were to identify and characterize bacteria associated with canker wilt of naranjilla, and to determine the importance of wounds in the dissemination and transmission of bacterial canker. Symptoms of this disorder consist of dieback and leaves showing incurvature which was diagnostic to confirm bacterial wilt. Collapse of leaf panel on Nicotiana tabacum confirmed that the bacterium is phytopathogenic. This bacterium showed positive serological tests for Clavibacter michiganensis subsp. michiganensis, positive Gram reaction, growth on NCP 88, (ELISA+ GRAM+ NCP 88 +) as well as re-isolation through Koch postulates in Solanum cheesmanii. Aerial wounds appear to be the most important means for dissemination. More research is needed about the molecular characterization of the bacterium, and potential for the bacterium to be transmitted to other solanaceous crops.
Keywords: emerging infectious crop diseases, Solanum quitoense, Solanum pectinatum, Solanum cheesmanii, Solanum hirsutum
RESUMEN
La marchitez bacteriana o cancro bacteriano de la naranjilla o lulo (Solanum quitoense y Solanum pectinatum) es una enfermedad con potencial para destruir completamente el cultivo. Los objetivos de este estudio fueron identificar y caracterizar la bacteria o bacterias asociadas al cancro bacteriano en naranjilla y determinar la importancia de las heridas en su transmisión y diseminación. Los síntomas de esta enfermedad consistieron en muerte descendente, y la curvatura de la nervadura principal que constituye un síntoma diagnóstico para la confirmación de marchitez bacteriana. El colapso del panel intervenal (propio de respuesta de hipersensibilidad) en Nicotiana tabacum permitió la confirmación de que la bacteria aislada era patogénica. Esta cepa bacteriana presentó serología positiva para Clavibacter michiganensis subsp. michiganensis, reacción Gram positiva, crecimiento en medio de cultivo semi-selectivo NCP 88 (ELISA+ GRAM+ NCP 88 +), así como re-aislamiento a partir de tejido de Solanum cheesmanii previamente inoculado para cumplir con los postulados de Koch. Heridas aéreas desarrolladas durante la poda con equipo de poda no desinfectado parece ser la forma principal de diseminación. Estudios adicionales son necesarios dirigidos hacia la caracterización molecular de la bacteria, y el potencial de diseminación de la bacteria hacia otras solanáceas.
Palabras clave: enfermedades infecciosas emergentes, Solanum quitoense, Solanum pectinatum, Solanum cheesmanii, Solanum hirsutum
INTRODUCTION
Naranjilla fruit, also known as lulo (Colombia), is produced using two botanical species: Solanum quitoense Lam. and Solanum pectinatum Dunal. Both species are endemic to the Andean region in South America. Its cultivation have economic importance for small growers in the mountainous and the Amazonian provinces of Ecuador. The crop presents serious phytopathological problems. Among them, those caused by plant pathogenic bacteria are very important.
In tomato (Solanum lycopersicum L.), bacterial canker caused by Clavibacter michiganensis have economic importance, too (Gitaitis, 1990). Clavibacter michiganensis presents Gram + reaction, coryneform cell morphology, cells are alone, in pairs, or in groups in forms of V or L (Koehm and Eggers-Schumacher, 1995).
Members of the genus Clavibacter have aerobic physiology of respiration, are generally found in vascular tissues, and show fastidious growth. There are four known subspecies, of them Clavibacter michiganensis subsp. michiganensis is associated with bacterial canker in tomato and other solanaceous crops (Gleason et al., 1991; EPPO, 2016). The mainly way for Clavibacter transmission is mechanically through wounds during pruning. It is highly infectious and yield losses could reach 100%.
Emerging infectious crop diseases are serious threats to a country. Crop pests or diseases that enter a new environment can establish and develop epidemics that may be difficult to control. Even in certain cases, these new pests or diseases cannot be controlled with disastrous results to agriculture production.
Preventing the introduction of new pests or diseases of quarantine organisms are a priority for agricultural research institutions and animal/plant inspection services. Crop Biosecurity depends on having appropriate steps to combat emerging threats. Therefore, early detection of foreign pests and diseases is crucial for maintaining an adequate phytosanitary status.
In 2008, Ochoa and Gallegos (Ochoa et al., 2016) made the initial detection of bacterial canker on Solanum quitoense and Solanum pectinatum in farmer fields. Additionally, Ochoa et al. (2016) mentioned that the spread of the disease is associated with grafting plants using infected knives. Bacterial canker is a new disease that could spread to other solanaceous crops. Therefore, studies aimed to identify and characterize the causal agent could prevent the dissemination of this disease. The objectives of this study were to identify and characterize bacteria associated with bacterial canker of naranjilla, and to determine the importance of wounds in the dissemination and transmission of bacterial canker.
MATERIALS AND METHODS
Stems of Solanum quitoense showing symptoms of bacterial canker (wilting, curvature of leaves, and cracked stems) were washed using tap water and treated with a sodium hypochlorite solution at 1% (v/v) during five minutes. Stems were air-dried and treated with a solution of ethanol 70% (v/v). Stems were washed with distilled sterile water and cut into 1-3 mm pieces to access the plant vessels. Segments with clear signs of vascular damage were placed inside 3 ml vials with distilled water and allowed to sit overnight. At the following day, suspension was serially diluted until 10-3. An aliquot of 100 µl was poured onto NCP 88 a semi-selective medium for genus Clavibacter (De la Cruz et al., 1992). Plates were incubated at 25 °C for 15 days.
Single colony isolates were transferred to a new plate with fresh NCP 88. Once the colony was sufficiently developed (a visible colony no longer stretched), it was transferred to a tube containing 3 ml of sterile distilled water. A drop of the solution was then transferred and evenly spread onto a new plate with NCP 88 using a Digralsky spatula. A petri plate containing the purified strain was opened in a laminar flow cabinet under aseptic conditions. A well-grown colony was taken and placed into a glass slide and Gram reaction was determined using KOH method (Buck, 1982). Strains that formed the mucilage thread were discarded due to the Gram – reaction as Clavibacter is Gram +.
ELISA test for Clavibacter michiganensis subsp. michiganensis was performed using the kit Agdia (Elkhart, Indiana, USA) according to the specifications of manufacturer. For this purpose, colonies of 48 -72 hours growth in NCP 88 were used. Colonies were diluted in sterile distilled water to achieve a concentration of 105 colony forming units (CFU) per ml (cfu ml-1).
Besides, 100 µl of the bacterial suspension were injected into intercellular panels of tobacco (Nicotiana tabacum L.) leaves using an insulin syringe. The presence of hypersensitive response was assessed at 48 and 72 hours after inoculation (Janse, 2005).
An experiment to evaluate the effect of wounds in the transmission and dissemination of the disease was designed. A potential strain of Clavibacter michiganensis subsp. michiganensis (ELISA+ Gram+ NCP 88+) was growth on NCP 88. Colonies of 48 - 72 hours of growth were diluted using distilled sterile water to reach a concentration of 108 cfu ml-1. The experiment consisted of three treatments (Table 1) arranged in a complete randomized design with five replications.
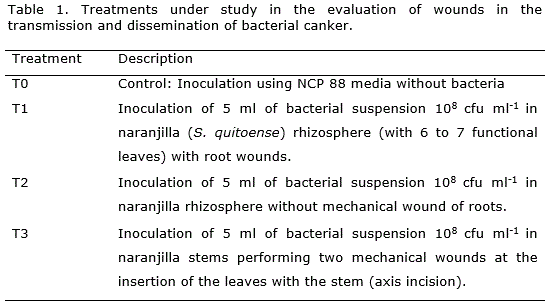
The experimental unit consisted of one pot with one seedling of naranjilla (Solanum quitoense). Additionally, treatment three (T3) was performed in a highly susceptible cultivar of Galapagos wild tomato (Solanum cheesmanii Riley) which has proven to be extremely susceptible to Clavibacter michiganensis subsp. michiganensis (Sen et al., 2013). Solanum quitoense and Solanum cheesmanii seeds were provided by the National Agricultural Research Institute germplasm collection (INIAP-DENAREF).
Specific methodology is summarized as follows:
T0- Control: Aseptically five milliliters of sterile NCP 88 liquid media were inoculated making incisions with the syringe needle of 10 ml capacity into the soil.
T1- Root damage: four incisions were made in the roots of the naranjilla plant. Five milliliters of 108 cfu ml-1 bacterial suspension were inoculated into the soil close to the incisions (rhizosphere).
T2: Non-root damage: five milliliters of 108 cfu ml-1 bacterial suspension were inoculated directly into the soil with the needle of a syringe of 10 ml capacity.
T3 - Aerial wound: the second functional leaf and the fourth leaf counted in descending order were removed using sterile scissors. Five milliliters of 108 cfu ml-1 bacterial suspension were inoculated making incisions with the needle of a 10 ml syringe. Stem incisions were made in order to put the bacteria in direct contact with the vascular tissue.
After inoculation, T0 to T3 were kept in a greenhouse at 17 to 20 °C. Humidity inside the greenhouse was between 70 to 80% RH.
The incubation period (days to onset of symptoms) was evaluated and the symptoms were described. The first visible symptoms expected in the inoculated seedlings are yellowing of leaves, apical wilting, and chlorosis and reduced growth compared to the control.
The scale to determine the parasitic ability of Clavibacter michiganensis on naranjilla (Solanum quitoense) was adapted from Sen et al. (2013). According to this scale: level 0 represents no symptoms; 1 represents the first leaf showing wilting and incurvatures; 2 represents more than one leaf showing wilting/incurvature, and less than 50% of leaves showing wilting, 3 represents 50% to 75% of leaves showing wilting, 4 represents more than 75% of the leaves showing wilting/incurvature, and 5 represents complete wilting and/or death of the plant. Scale values per experimental unit were analyzed using one-way ANOVA. Analysis was carried out using INFOSTAT software (Universidad de Cordoba, Argentina).
Numeric codes for bacteria were assigned to each strain (culture collection of the National Department of Plant Protection- INIAP). Colonies were preserved in distilled water (Iacobellis and deVay, 1986) at room temperature.
RESULTS AND DISCUSSION
Colonies from plant material with disease symptoms were obtained in NCP 88 medium. It predominantly showed entire margins, white to cream colored, slightly raised and mucoid. After 10 to 12 days of incubation, the colonies changed to pale yellow. White-cream colonies on NCP 88 is a distinctive characteristic of Clavibacter versus other pink or orange Gram + bacteria (De la Cruz et al., 1992).
ELISA test was positive with a value of the potential strain of 0.51 and 0.56 points of absorbance for Clavibacter michiganensis subsp. michiganensis positive control. Previous work carried out by Ochoa and Gallegos (2012) detected a positive serological reaction of bacterial isolates from naranjilla collected in the Amazonia of Ecuador.
The presence of necrosis or hypersensitivity reaction in the inoculation leaf panel indicates pathogenicity ability of the bacterium isolated from naranjilla plants (Figure 1 c).
In Solanum quitoense, incurvature of the leaves (Figure 1 e) is a diagnostic symptom that allows the differentiation of vascular wilt caused by Fusarium oxysporum versus bacterial canker caused by Clavibacter michiganensis subsp. michiganensis. Fusarium oxysporum does not produce incurvature of the leaves when it disrupts the vascular tissue of naranjilla (Ochoa and Ellis, 2002).
In Solanum cheesmanii at 18 days after inoculation, no disease symptoms were observed. Our results are different from those reported by Sen et al. (2013), who found early symptoms at 18 days after inoculation. The reasons for the late detection of symptoms in a highly susceptible plant such as S. cheesmanii is unknown and could be related to a difference in strain virulence. In this regard, Sen et al. (2015) mentioned that Clavibacter shows a great variability in virulence in tomato.
At 25 days after inoculation, wilting was observed in the immediate leaf of the first inoculation (leaf 2) on plants of T3 (removing two leaves and aerial inoculation with the syringe).
Infection proceeded and at 42 days after inoculation wilting was observed in six leaves of the plant of T3, but this was not complete and wilting was located on the side where the inoculations were performed. At 56 days after inoculation the plant Solanum cheesmanii presented total collapse of the vascular system with generalized wilting and stem canker (Figure 1 a-b).

In Solanum quitoense, initial symptoms were detected at 40 days after inoculation in the T3 treatment (Figure 1 d –e ). In naranjilla, incurvature of the leaves (Figure 1 e).
In T1 (root damage and subsequent inoculation), early symptoms were observed at 60 days after inoculation (Figure 1 f). T0 (control), as well as T2 (no-wound and inoculation), did not present symptomatology during this study (Table 2, Figure 1 f). Complete collapse of the naranjilla plant was reached after 110 days of inoculation in the treatment T3 (aerial inoculation) (Figure 1 f center). These results suggest that mechanical wound is of paramount importance for the dissemination and transmission of bacterial canker by Clavibacter michiganensis subsp. michiganensis in naranjilla. Tancos et al. (2013) demonstrated that Clavibacter michiganensis subsp. michiganensis infects tomato flowers and invade seedlings both systemically through the xylem or externally via fruit lesions, highlighting the importance of multiple entry routes of Clavibacter for bacterial wilt. In tomato, it is well known that seeds are the primary source for Clavibacter michiganensis infections (Tsiantos, 1987; Milijašević-Marčić et al., 2012; Tancos et al., 2013). However, in a crop best produced by grafting such as naranjilla, the importance of mechanical transmission can overcome the importance of seedlings for outbreaks.
Based on the result showed on table 2, the most effective method to disseminate Clavibacter michiganensis subsp. michiganensis on naranjilla could be aerial wounds. This information is of vital importance due to the mechanical pruning that growers normally perform. Solanum cheesmanii inoculated using a pure strain which presented positive reaction to the ELISA test for Clavibacter michiganensis subsp. michiganensis (ELISA+ Gram + NCP88 +) showed symptoms of disease at 40 days, and the same phenotype of bacterium (ELISA+ Gram + NCP88 +) was re-isolated from diseased plant tissue.
CONCLUSIONS
Based on the results such as the collapse of leaf panel on Nicotiana tabacum (confirming that the bacterium is phytopathogenic), positive serological tests, positive Gram reaction, growth on NCP 88 medium, and the presence of the bacterium strain (inoculated in Solanum cheesmanii), after presence of diagnostic symptomatology (incurvature of leaves), it was detected bacterial canker of naranjilla (S. quitoense) caused by Clavibacter michiganensis subsp. michiganensis. More research is needed about the molecular characterization of the bacterium, aggressiveness, and potential of the bacterium to be transmitted to other solanaceous crops.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge technical support by the Ecuadorian Agency for Quality Assurance in Agriculture (AGROCALIDAD) and the Autonomous National Institute of Agricultural Research (INIAP), Cutuglahua, Ecuador.
Conflict of interest None declared.
REFERENCES
Buck J (1982) Non-staining (KOH) method for determination of gram reactions of marine bacteria. Appl Environ Microbiol 44(4): 992–993
De la Cruz A, Wiese M, Schaad N (1992) A semi-selective agar medium for isolation of Clavibacter michiganensis subsp. sepedonicus from potato tissues. Plant Dis 76(8): 830-834; doi:10.1094/PD-76-0830
EPPO (2016) Clavibacter michiganensis subsp. michiganensis. Bulletin OEPP 46(2): 202–225; doi:10.1111/epp.12302
Gitaitis R (1990) Induction of a hypersensitive like reaction in four-o'clock by Clavibacter michiganensis subsp. michiganensis. Plant Dis 74(1): 58-60; doi:10.1094/PD-74-0058
Gleason M, Braun E, Carlton W, Peterson R (1991) Survival and dissemination of Clavibacter michiganensis subsp. michiganensis in tomatoes. Phytopathology 81(12): 1519-1523; doi:10.1094/Phyto-81-1519
Iacobellis N, DeVay J (1986) Long-term storage of plant-pathogenic bacteria in sterile distilled water. Appl Environ Microbiol 52(2): 388-389
Janse JD (2005) Phytobacteriology: principles and practice. CABI Pub, Wallingford UK; ISBN: 9781845930356
Koehm B, Eggers-Schumacher G (1995) Rapid and simple detection of plant pathogens by reverse passive haemagglutination (RPH): detection of the bacteria Clavibacter michiganensis (ssp. sepedonicus) and Erwinia carotovora (ssp. carotovora) with RPH in potato tubers. Z Pflanzenkr Pflanzenschutz 102(1): 58–62
Milijašević-Marčić S, Gartemann K H, Frohwitter J, Eichenlaub R, Todorović B, Rekanović E, Potočnik I (2012) Characterization of Clavibacter michiganensis subsp. michiganensis strains from recent outbreaks of bacterial wilt and canker in Serbia. European journal of plant pathology 134(4): 697-711; doi:10.1007/s10658-012-0046-x
Ochoa JB, Ellis MA (2002) Seed transmission of Fusarium oxysporum in common naranjilla (Solanum quitoense) in Ecuador, Plant health progress. Available in: http://www.plantmanagementnetwork.org/pub/php/brief/naranjillavw/. Accessed 13/09/2017
Ochoa J, Gallegos P (2012) Prácticas de Manejo Integrado de Plagas desarrolladas por el INIAP para el manejo del cultivo de naranjilla Informe Técnico Anual 2013 del Proyecto INIAP-IPM CRSP. INIAP, Ecuador
Ochoa J, Clements C, Barrera V, Dominguez J M, Ellis M A, Alwang J (2016) IPM Packages for Naranjilla: Sustainable Production in an Environmentally Fragile Region. En: Rangaswamy M, Heinrichs E A (Eds). Integrated Pest Management of Tropical Vegetable Crops, pp. 209-221. Springer, Netherlands; ISBN: 978-94-024-0922-2
Sen Y, van der Wolf J, Visser RG, van Heusden S (2015) Bacterial canker of tomato: current knowledge of detection, management, resistance, and interactions. Plant Disease 99 (1): 4-13; doi:10.1094/PDIS-05-14-0499-FE
Sen Y, Feng Z, Vandenbroucke H, van der Wolf J, Visser R, Van Heusden A (2013) Screening for new sources of resistance to Clavibacter michiganensis subsp. michiganensis (Cmm) in tomato. Euphytica 190(2): 309-317; doi:10.1007/s10681-012-0827-5
Tancos M A, Chalupowicz L, Barash I, Manulis-Sasson S, Smart C D (2013) Tomato fruit and seed colonization by Clavibacter michiganensis subsp. michiganensis through external and internal routes. Applied and environmental microbiology 79(22): 6948-6957; doi:10.1128/AEM.02495-13
Tsiantos J (1987) Transmission of bacterium Corynebacterium michiganense pv. michiganense by seeds. Journal of Phytopathology 119(2): 142-146; doi:10.1111/j.1439-0434.1987.tb00476.x
Recibido: 16-10-2017
Aceptado: 30-11-2017
Copyright (c) 2018 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
