Artículo original
Biotecnología Vegetal Vol. 18, No. 3: 151-159, julio - septiembre, 2018
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Antioxidant enzymes preserving coffee quality in refrigerate environment
Enzimas antioxidantes preservan la calidad del café en ambiente refrigerado
Giselle Figueiredo de Abreu1, Sttela Dellyzete Veiga Franco da Rosa2, Marcelo Ribeiro Malta3, Aline da Consolação Sampaio Clemente4, Amanda Lima Vilela4, Rucyan Walace Pereira4
1Departamento de Ingenieria, Universidad Federal de Lavras. Campus Universitário, Caixa Postal 3037. Lavras. MG. Brasil. CP 37200-00. e-mail: gfigueiredoabreu@hotmail.com
2Embrapa Café, Universidad Federal de Lavras. Campus Universitário, Caixa Postal 3037. Lavras. MG. Brasil. CP 37200-000.
3Empresa de Investigación Agropecuaria de Minas Gerais, Universidad Federal de Lavras. Campus Universitário, Caixa Postal 3037. Lavras. MG. Brasil. CP 37200-000.
4Departamento de Agricultura, Sector de semillas, Universidad Federal de Lavras. Campus Universitário, Caixa Postal 3037. Lavras. MG. Brasil. CP 37200-000.
ABSTRACT
Post-harvest phases may cause biochemical and physiological changes with direct effects on coffee (Coffea sp.) grains quality during its storage. The aim of this study was to determine the relevance of antioxidant isoenzyme expression on the coffee grains quality undergoing different types of processing and storage conditions. Coffea arabica L. ripe fruits was harvested, part of them was dry processed and another part was wet processing. Immediately, the coffee was dried. After drying, some of the grains were hulled and the other part was maintained unhulled. After that, the grains were stored under refrigerate controlled conditions (10 °C and 50% relative humidity) or at 25 °C without relative humidity control for a period of 12 months. Enzymatic expression of catalase, esterase, peroxidase, and alcohol dehydrogenase enzymes was evaluated in the coffee grains in these storage conditions by electrophoretic analysis. The results were compared to the sensorial and physiological profiles of the samples. It was found that expression of enzymes of the antioxidative process is associated with changes in the quality of coffee grains. Natural coffee obtained by dry processing is more sensitive to biochemical changes than wet-processed coffee. A refrigerated environment has a beneficial effect for preserving the coffee grains showed by higher expression of catalase, peroxidase, and alcohol dehydrogenase enzymes after six months of storage in these conditions. The protection of the endosperm by the presence of the pericarp in natural coffee or the endocarp in fully-washed coffee is beneficial to maintenance of quality and is related to expression of antioxidant enzymes.
Keywords: alcohol dehydrogenase, catalase, Coffea arabica L., esterase, peroxidase
RESUMEN
Las etapas de la poscosecha pueden causar alteraciones bioquímicas y fisiológicas con efectos directos en la calidad de los granos de cafeto (Coffea spp.) durante su almacenamiento. El objetivo de este trabajo fue determinar la relevancia de la expresión de isoenzimas del proceso antioxidante, en la conservación de la calidad de los granos sometidos a diferentes tipos de procesamiento y condiciones de almacenamiento. Los frutos de Coffea arabica L. fueron cosechados en estado de maduración cereza y secados, luego de ser beneficiados por vía seca y por vía húmeda. Después del secado, parte de los granos fue trillada y otra parte conservada en coco o en pergamino. Luego, los granos fueron almacenados en condiciones controladas de aire refrigerado (10 °C y humedad relativa del 50%) o a 25 °C sin control de la humedad relativa, durante un periodo de 12 meses. La expresión enzimática de las enzimas catalasa, esterasa, peroxidasa y alcohol deshidrogenasa fue evaluada en los granos de café a lo largo del almacenamiento por medio de un análisis electroforético. Estos resultados fueron comparados con el perfil sensorial y fisiológico de las muestras. Se constató que la expresión de las enzimas del proceso antioxidante, está asociada con alteraciones en la calidad de los granos de café. El café natural obtenido por beneficio mediante secado, es más sensible a las alteraciones bioquímicas del beneficiado por vía húmeda. El efecto beneficioso de la refrigeración del aire de almacenamiento en la preservación de la calidad del café, es evidenciado por la mayor expresión de las enzimas estudiadas después de seis meses. La expresión de las enzimas del proceso antioxidante, está asociada al efecto protector del pericarpio y endocarpio.
Palabras clave: alcohol deshidrogenasa, catalasa, Coffea arabica L., esterasa, peroxidasa
INTRODUCTION
In post-harvest, coffee (Coffea spp.) grains are subject to numerous physical, physiological and biochemical changes, which may have a negative effect on sensorial quality (Borém et al., 2013; Saath et al., 2014; Selmar et al., 2014; Taveira et al., 2014; Ribeiro et al., 2016). These events could cause grain deterioration, induce the production of reactive oxygen species (ROS), enzyme degradation and inactivation, reduction in respiratory activity, and loss of cell membrane integrity (Vidigal et al., 2009; Sharma et al., 2012; Coelho et al., 2017).
Some environmental stresses lead to production of ROS that could cause oxidative damage to the tissues depends on the delicate equilibrium between the production and their scavenging (Dussert et al., 2006). The progress of the grains deterioration under stress conditions, as occurs during storage, can be detected by changes in expression of enzymatic systems (Dussert et al., 2006; Sharma et al., 2012). Therefore, biochemical analyses can provide deterioration markers and could contribute to ensure preservation of coffee grains quality. The changes in the activity of certain enzymes, depending on the intensity, time of exposure and the type of stress (Moussa and Abdel-Aziz, 2008; Han et al., 2009).
Coffee fruits are beneficiate by mechanical processing operations for removing the pericarp, in the case of natural coffee, and the endocarp in fully-washed coffee. These operations can cause cracks or latent damage that will later show up, during storage. The mechanical damage lead to destruct cell membranes and provoke cell disorganization with furthering reduction in quality (Selmar et al., 2008).
Usually, coffee grains are stored after processing and drying. The large-scale storage carried out in conventional models using jute bags in an environment without temperature and relative humidity control. Therefore, grains are exposed to stress conditions. An alternative to reduce or avoid the effect of stress on grains quality is the storage in a refrigerate environment which has shown positive results (Araujo et al., 2008; Rigueira et al., 2009; Abreu et al., 2017). Nevertheless, the role of antioxidant enzymes in this storage condition not have been fully elucidate.
The aim of this paper was to determine the relevance of antioxidant isoenzyme expression on the coffee grains quality undergoing different types of processing and storage conditions.
MATERIALS AND METHODS
Plant material
Fruits of Coffea arabica L. cv. Catuaí Amarelo harvested from an experimental farm of Fundação Procafé in the municipality of Varginha, MG, Brazil.
Fruit processing
Fruits were harvested selectively in the ripe stage of maturation (cherry) and then it were washed to remove floaters fruits malformed or attacked by coffee borer and other impurities before processing. Part of the selected fruits were immediately submitted to drying (natural coffee) and another part was subjected to mechanical pulping and removal of mucilage through fermentation in water for 24 hours (fully-washed coffee) and then, drying.
Grain processing
The grains (seeds) were dried on suspended polyethylene screens and turned 12 times per day until reaching 30% moisture content wet basis (wb) for natural coffee and 25% wb for fully-washed coffee. In the half-dried state, the grains were transferred to fixed-bed mechanical driers where the temperature was maintained at 35°C and constantly monitored using a mercury thermometer until the grains reaching 11% wb moisture content.
After drying, a part of the grains was hulled in the pulper (Palini & Alves, model PA-DESC), connected to the dryer, for cleaning, hulling, and ventilation, and the other part was not hulled.
Grains storage
The coffee grains were packed in Jutex® polypropylene bags and stored for 12 months in two environments: in a climate-controlled cooling chamber (10 °C, 50% RH) and in a storage room at constant 25 °C, without control of relative humidity. At evaluation time, the coffee grains stored without hulling were hulled manually to avoid damage.
Isoenzymatic analysis
Before and after three, six, and twelve months of storage, samples were collected from all the treatments, and it were kept in a deep freezer (-80 °C) until performing electrophoretic analysis of isoenzymes.
The extraction of the esterase (EC 3.1.1.2), catalase (EC 1.11.1.6), and alcohol dehydrogenase (EC 1.1.1.1) enzymes was performed with buffer 0.2 M Tris HCl, pH 8.0 and 0.1% β-mercaptoethanol at the rate of 320 µl per 100 mg of grains. The mixture was homogenized in a vortex and kept for 60 minutes at 4 °C in a refrigerator, followed by centrifuging at 14 000 rpm for 60 minutes at 4 °C.
In the case of peroxidase (EC 1.11.1.7) enzyme the extraction was made in phosphate buffer (0.034 M disodium phosphate, 0.2 M sucrose, 2.56% PVP40, 3M DTT, 5.7 mM L-ascorbic acid, 2.5 mM sodium borate, 1% PEG 6000, 0.002% β-mercaptoethanol) and 2 g PVP 40 (polyvinylpyrrolidone) antioxidant.
Electrophoresis analysis was performed in a polyacrylamide gel system with a 7.5% separator gel and 4.5% concentrator gel. The gel/electrode system used was Tris glycine, pH 8.9. An aliquot of 50 μl of the samples supernatant was applied in the gel, and the electrophoresis was carried out at 120 V for 5 hours. The gels were revealed for the enzymes esterase, catalase, alcohol dehydrogenase, and peroxidase, as described by Alfenas (2006). Evaluation of the enzymatic patterns was carried out in a qualitative manner according to the presence and intensity of the bands, using a transilluminator.
The results of isoenzymes analysis were compared to sensorial analysis of the coffee and physiological profile of the grains, evaluated by the germination test.
Sensory analysis
The sensory analysis followed the protocol set by the Specialty Coffee Association (SCA) for sensory evaluation of specialty coffees (Lingle, 2011). According to this methodology, scores are attributed to fragrance/aroma, acidity, body, flavor, aftertaste, sweetness, uniformity, clean cup, balance and overall impression.
Moderately light roasting was carried out in 100g of coffee beans (> 16 mesh). Temperature was monitored to assure that the roasting time was not shorter than 8 min or longer than 12 min. The roasting sample pattern of the first storage period was stored in order to standardize the roasting process and to prevent it from influencing the evaluation of the tasters. Such pattern was used as reference during the roasting process in each evaluation period. All samples were roasted at least 12 hours prior to tasting. The final sensory evaluation results encompassed the sum of all attributes.
Germination test
Performed in four replicates of 50 grains without the parchment, on germitest-type paper sheets moistened with distilled water, in a germinator regulated at 30 °C. The evaluation was performed at 30 days after sowing, expressing the results in percentage of normal seedlings, based on the recommendations of the RAS (Brasil, 2009).
RESULTS AND DISCUSSION
Electrophoretic analysis showed that in natural coffee the expression of catalase was higher in comparison to fully-washed coffee (Figure 1). In other studies, similar results are informed and coinciding with poor physiological quality in wet-processed coffee grains compared to dry-processed (Taveira et al., 2012). Over the storage period, reduction in expression of the catalase enzyme was observed, especially in natural coffee stored at 25 °C. These results may be associated with the level of deterioration since in these treatments low physiological and sensorial performance is observed throughout storage when compared to grains that are wet processed (Figure 2, Figure 3). The results indicated that storage of natural coffee under 25 °C can propel or accelerate the grains deterioration process.
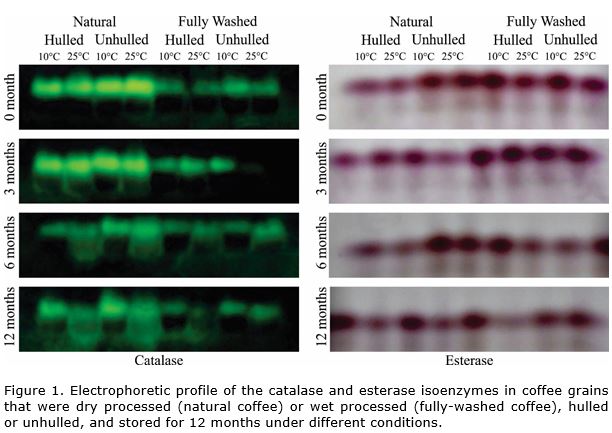
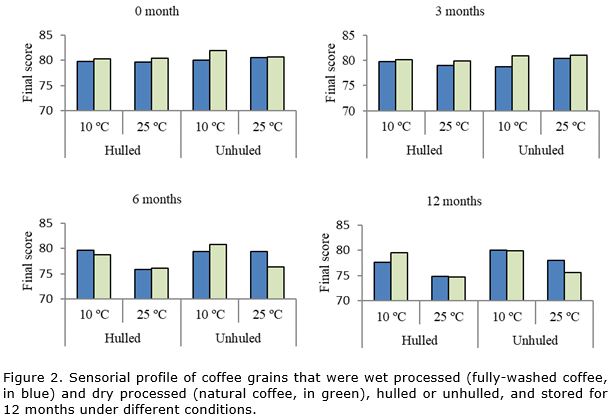
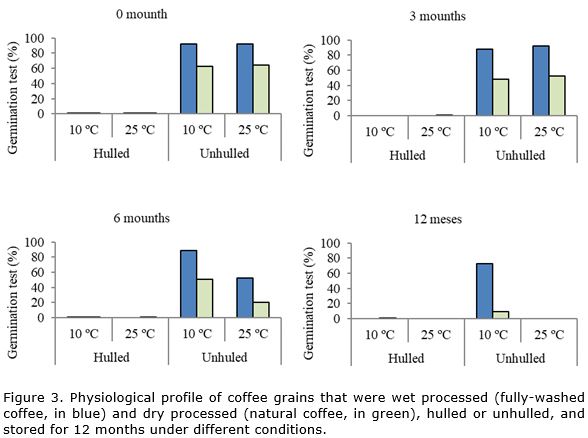
In relation to the effect of hulling, the mechanically hulled coffee exhibited lower expression of catalase compared to the unhulled grains over the storage period. Upon associating this result with the physiological profile of the grains, it can be noted that the germination of the hulled and stored grains was null, as of the first period of evaluation. In addition, in the sensorial profile, a greater reduction was also noted in the quality of the hulled coffees stored in the two types of processing (Figure 3).
Thus, the lower expression of catalase can be explained by the fact that these grains are already in an advanced state of deterioration, probably by the presence of mechanical damage caused by hulling, affecting enzyme synthesis (Sharma et al., 2012). As for grains stored without hulling, the presence of the pericarp may have favored preservation of quality throughout storage (Selmar et al., 2008; Rendón et al., 2014). It should be emphasized that these grains did not exhibit mechanical damage since it were hulled manually, before the evaluation.
For the fully-washed coffee grains, differences in expression of the catalase enzyme were not observed, possibly because wet-processed coffee is less sensitive to changes caused by the steps of post-harvest processing compared to natural coffee (Taveira et al., 2012; Malta et al., 2013; Saath et al., 2014).
In relation to storage conditions (10 °C, RH 50%, 25 °C without control RH), the biggest differences were observed as six months of storage, with higher enzymatic expression of catalase in the grains stored at 25°C, without control of RH, in comparison to coffee grains stored in a cooled environment (10 °C, 50% RH). However, this situation was more evident in the dry-processed grains, which was also observed in the results of sensorial analysis (Figure 3). It is known that inadequate storage can lead to stress conditions for grains, favoring the deterioration process and changing enzyme expression (Dussert et al., 2006). Temperature and relative humidity conditions are extremely important for evolution of the deterioration process in coffee grains during storage (Nobre et al., 2007; Borém et al., 2008; Abreu et al., 2017).
In general, for the esterase enzyme, higher enzyme expression was observed in coffee grains before storage (time zero of evaluation) or after the first months of storage, with reduction in expression of this enzyme throughout storage (Figure 1), just as was observed in evaluation of sensorial quality (Figure 2). In the same way, in other species had been observed higher intensity of the bands and activity of the esterase enzyme before storage and it decline as the deterioration process advanced throughout storage (Padilha et al., 2001; Santos et al., 2004).
Differences were observed in the electrophoretic profile of the esterase enzyme in relation to the type of processing, with higher expression in unhulled natural coffee at the beginning of storage compared to hulled grains. The result of enzyme expression observed in unhulled grains stored at 10 °C after six months of storage demonstrated that lower air temperature and the presence of the pericarp protecting the grains has a beneficial effect.
As for the wet-processed grains (fully-washed), at the beginning of storage, a similar pattern of esterase expression among the treatments can be observed. However, after six months of storage, a reduction in enzymatic expression was observed in hulled coffee grains stored at 25 °C in relation to the other treatments. In contrast, there was no change in enzyme expression when the fully-washed coffee was stored in cooled air (10 °C), regardless of whether the coffee was hulled.
The grains unhulled and stored at a 10 °C showed higher expression of the esterase enzyme during the storage, also exhibited better physiological performance (Figure 2). The results are in agreement with the reports of Brandão et al. (2002) that reported a decrease in the number of bands and intensity of the esterase enzyme with a loss of viability of coffee seeds.
In relation to the peroxidase enzyme, an increase in enzymatic expression was observed throughout storage time (Figure 4). This enzyme is associated with oxidation by peroxides, promoting cell detoxification upon removing hydrogen peroxide. This is an important defense mechanism in plant organisms (Moussa and Abdel-Aziz, 2008; Han et al., 2009).
Before storage, lower expression of peroxidase was observed in fully-washed coffee that was mechanically hulled in comparison to the other treatments.
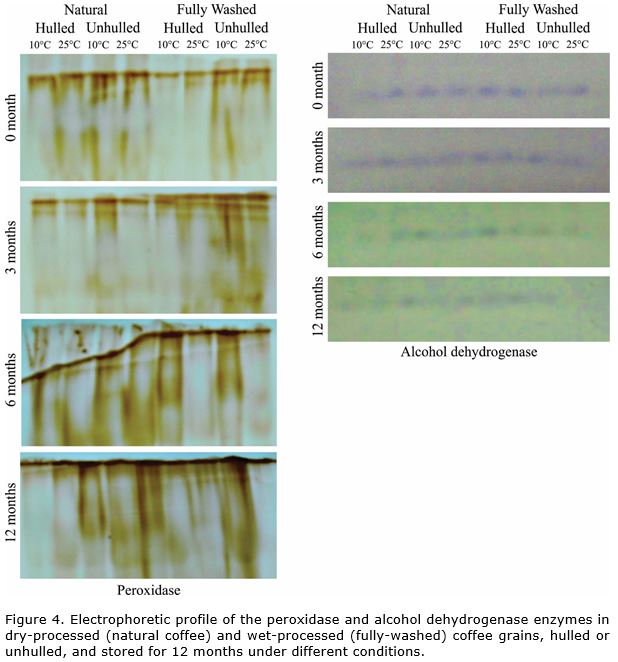
In fully-washed coffee grains, higher enzymatic expression of peroxidase was observed after sixth month of storage under cool conditions (10 °C and 50% RH) in relation to grains stored at 25 °C. However, in natural coffee, higher expression of the enzyme occurred in unhulled (dry cherry) grains, stored at 10 °C and 50% RH. In both types of processing, the grains that had higher peroxidase expression also had better physiological performance (Figure 2, Figure 3).
Similar results were informed by others authors, that observed higher enzymatic expression of peroxidase in grains with superior physiological quality since the loss of its activity can make the seeds more sensitive to the effects of O2 and free radicals on unsaturated fatty acids of the membrane (Taveira et al., 2012; Saath at al., 2014). This situation leads to degeneration of membranes and physiological damage to grains.
In the case of alcohol dehydrogenase (ADH) enzyme, a reduction on its expression was observed (Figure 4), coinciding with reduction in physiological quality (Figure 2) and sensorial quality (Figure 3) of the grains during the storage period in all the treatments. The result confirmed the association between accumulation of acetaldehyde and progression of the deterioration process during storage (Zhang et al., 1994). ADH enzyme acts in the anaerobic metabolism of plants, promoting a reduction of acetaldehyde to ethanol (Buchanan et al., 2015).
Upon comparing the wet-processed grains and dry-processed grains, it can be observed that natural coffee, with lower physiological quality in relation to fully-washed coffee (Figure 2), also exhibits lower expression of alcohol dehydrogenase (Figure 4). The effects of the type of processing are observable after sixth month of storage. This shows the greater sensitivity of natural coffee to storage conditions and, therefore, to the deterioration process.
Electrophoretic analysis revealed that after 6 months of storage, the ADH enzyme showed an increased expression in grains stored under 10°C and 50% RH. In addition, after 12 months of storage, the enzymatic expression was higher in grains unhulled and stored under conditions more favorable to maintaining the quality. In both situations, the grains that had higher expression of ADH are also those that had better physiological results (Figure 2) and better sensorial quality (Figure 3). These results corroborate those found by other authors, that observed reduction in enzymatic expression of ADH together with a decline in grains physiological performance (Vidigal et al., 2009).
CONCLUSIONS
Expression of antioxidant enzymes is associated with deterioration of coffee grains during the storage. In addition, coffee grains with dry processing are more sensitive to biochemical changes in the deterioration process than those submit to wet processing, evidenced by higher expression of the catalase and alcohol dehydrogenase enzymes in wet-processes grains. Besides, a refrigerated environment has a beneficial effect for preserving the coffee grains showed by higher expression of catalase, peroxidase, and alcohol dehydrogenase enzymes after six months of storage in these conditions. The protection of the endosperm by the presence of the pericarp in natural coffee or the endocarp in fully-washed coffee is beneficial to maintenance of quality and is related to expression of antioxidant enzymes.
ACKNOWLEDGMENTS
Our thanks to FAPEMIG, CNPq, CAPES, Embrapa, and Consórcio Pesquisa Café for financial support in research.
Conflict of interest
The authors not declared conflict of interest.
REFERENCES
Alfenas AC (2006) Eletroforese e marcadores bioquímicos em plantas e microrganismos. UFV, Viçosa
Abreu GF, Rosa SDVF, Cirillo MA, Malta MR, Clemente ACS, Borém FM (2017) Simultaneous optimization of coffee quality variables during storage. Bras Eng Agríc Ambiental 21(1): 56–60; doi:10.1590/1807-1929/agriambi.v21n1p56-60
Araujo RF, Araujo EF, Cecon PR, Sofiatti V (2008) Conservação de sementes de café (Coffea arabica L.) despolpado e não despolpado. Revista Brasileira de Sementes 30(3): 71–78; doi:10.1590/S0101-31222008000300010
Borém FM, Nobre GW, Fernandes SM, Pereira RGFA, Oliveira PD (2008) Avaliação sensorial do café cereja descascado, armazenado sob atmosfera artificial e convencional. Ciência e Agrotecnologia 32(6): 1724–1729; doi:10.1590/S1413-70542008000600007
Borém FM, Ribeiro FC, Figueiredo LP, Giomo GS, Fortunato VA, Isquierdo EP (2013) Evaluation of the sensory and color quality of coffee beans stored in hermetic packaging. Journal of Stored Products Research 52: 1–6; doi:10.1016/j.jspr.2012.08.004
Brandão DS, Vieira MGGC, Guimarães RM, Hilhorst HWM (2002) Tolerância à dessecação de sementes de cafeeiro (Coffea arabica L.). Revista Brasileira de Sementes 24(2): 17–23; doi:10.1590/S0101-31222002000100004
Brasil (2009) Regras para análise de sementes (RAS) Mapa/Assessoria de Comunicação Social. Ministério da Agricultura Pecuária e Abastecimento, Brasília
Buchanan BB, Gruissem W, Jones RL (2015) Biochemistry & molecular biology of plants. Wiley-Blackwell, London; ISBN: 9780470714218
Coelho SV, Rosa SD, Fernandes JS (2017) Seed. Science & Technology 45(3): 1-12; doi:10.15258/sst.2017.45.3.15
Dussert S, Davey MW, Laffargue A, Doulbeau S, Swennen R, Etienne H (2006) Oxidative stress, phospholipid loss and lipid hydrolysis during drying and storage of intermediate seeds. Physiologia Plantarum 127(2): 192–204; doi:10.1111/j.1399-3054.2006.00666.x
Han C, Liu Q, Yang Y (2009) Short-term effects of experimental warming and enhanced ultraviolet-B radiation on photosynthesis and antioxidant defense of Picea asperata seedlings. Plant Growth Regulation 58(2): 153–162; doi:10.1007/s10725-009-9363-2
Lingle TR (2011) The Coffee Cupper’s Handbook: Systematic Guide to the Sensory Evaluation of Coffee’s Flavor. Coffee Association of America, Washington
Malta MR, Rosa SDVF, Lima PM, Fassio, LO, Santos JB (2013) Alterações na qualidade do café submetido a diferentes formas de processamento e secagem. Engenharia Na Agricultura 21(5): 431–440; doi:10.13083/reveng.v21i5.450
Moussa HR, Abdel-Aziz SM (2008) Comparative response of drought tolerant and drought sensitive maize genotypes to water stress. Australian Journal of Crop Science 1(1): 31–36
Nobre GW, Borém FM, Fernandes SM, Pereira RGFA (2007) Alterações químicas do café cereja descascado durante o armazenamento. Coffee Science 2(1): 1–9; doi:10.25186/cs.v2i1.33
Padilha L, Vieira MGGC, Von Pinho EVR, Carvalho MLM (2001) Relação entre o teste de deterioração controlada e o desempenho de sementes de milho em diferentes condições de estresse. Revista Brasileira de Sementes 23(1): 198–204; doi:10.17801/0101-3122/rbs.v23n1p198-204
Rendón MY, Salva TJG, Bragagnolo N (2014) Impact of chemical changes on the sensory characteristics of coffee beans during storage. Food Chemistry, 147: 279-286; doi:10.1016/j.foodchem.2013.09.123
Ribeiro DE, Borém FM, Cirilo MA, Prado MVB, Ferraz VP, Alves HMR, Taveira JHS (2016) Interaction of genotype, environment and processing in the chemical composition expression and sensorial quality of Arabica coffee. African Journal of Agricultural Research 11(27): 2412–2422; doi:10.5897/AJAR2016.10832
Rigueira RJA, Lacerda Filho AF, Volk MBS, Cecon PR (2009) Armazenamento de grãos de café cereja descascado em ambiente refrigerado. Engenharia Na Agricultura 17(4): 323–333; doi:10.13083/reveng.v17i4.75
Saath R, Broetto F, Biaggioni MAM, Borém FM, Rosa SDVF, Taveira JHS (2014) Activity of some isoenzymatic systems in storage coffee grains. Ciência E Agrotecnologia 38(1): 15–24; doi:10.1590/S1413-70542014000100002
Santos CMR, Menezes NL, Villela FA (2004) Alterações fisiológicas e bioquímicas em sementes de feijão envelhecidas artificialmente. Revista Brasileira de Sementes 26(1): 110–119; doi:10.1590/S0101-31222004000100017
Selmar D, Bytof G, Knopp SE (2008) The storage of green coffee (Coffea arabica): Decrease of viability and changes of potential aroma precursors. Annals of Botany 101(1): 31–38; doi:10.1093/aob/mcm277
Selmar D, Kleinwächter M, Bytof G (2014) Metabolic responses of coffee beans during processing and their impact on coffee flavor. In: Schwan FR, Fleet HG (Eds). Cocoa and Coffee Fermentations, pp. 73–81. CRC Press, Boca Raton; doi:10.1016/B978-0-12-409517-5.00009-7
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 26(1): 1–26; doi:10.1155/2012/217037
Taveira JHS, Borém FM, Figueiredo LP, Reis N, França AS, Harding SA, Tsai CJ (2014) Potential markers of coffee genotypes grown in different Brazilian regions: A metabolomics approach. Food Research International 61: 75–82; doi:10.1016/j.foodres.2014.02.048
Taveira JHS, Rosa SDVF, Borém FM, Giomo GS, Saath R (2012) Perfis proteicos e desempenho fisiológico de sementes de café submetidas a diferentes métodos de processamento e secagem. Pesquisa Agropecuaria Brasileira 47(10): 1511–1517; doi:10.1590/S0100-204X2012001000014
Vidigal DS, Dias DCFS, Von Pinho EVR, Dias LAS (2009) Alterações fisiológicas e enzimáticas durante a maturação de sementes de pimenta (Capsicum annuum L.). Revista Brasileira de Sementes 31(2): 129–136; doi:10.1590/S0101-31222009000200015
Zhang M, Maeda Y, Furihata Y, Nakamaru Y, Esashi Y (1994) A mechanism of seed deterioration in relation to the volatile compounds evolved by dry seeds themselves. Seed Science Research 4(1): 49–56; doi:10.1017/S0960258500001999
Recibido: 10-04-2018
Aceptado: 24-06-2018
Copyright (c) 2018 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
