Artículo original
Biotecnología Vegetal Vol. 19, No. 1: 15 - 24, enero - marzo, 2019
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Potato purple top, lethal wilt of oil palm, and papaya twisted neck syndrome: Phytoplasma-associated diseases in Ecuador
Punta morada de la papa, Marchitez letal de la palma aceitera y sSíndrome de cuello virado de la papaya: enfermedades asociadas a fitoplasmas en Ecuador
Carlos Bolanos1, Patricio Gallegos1, Jose B Ochoa1, Maria Insuasti1, Veronica Bonilla2, Jorge Rivadeneira3, Paul Comina3, Xavier Cuesta3
1 National Department of Plant Protection, Instituto Nacional de Investigaciones Agropecuarias-INIAP. Panamericana Sur km 1 vía Tambillo. Mejía. Pichincha. Ecuador. CP 170401.
2 National Department of Biotechnology, Instituto Nacional de Investigaciones Agropecuarias-INIAP. Panamericana Sur km 1 vía Tambillo. Mejía. Pichincha. Ecuador. CP 170401.
3National Program of roots and tubers - Potato, Instituto Nacional de Investigaciones Agropecuarias-INIAP. Panamericana Sur km 1 vía Tambillo. Mejía. Pichincha. Ecuador. CP 170401.
*Author for correspondence e-mail: cbolanos@huskers.unl.edu
ABSTRACT
Phytoplasma are wall-less bacteria limited to the phloem vessels in higher plants. Diseases associated with phytoplasma, in the past, have not been a serious problem in Ecuador. Nevertheless, for climate change effects, their importance has been increasing suddenly. This research was focused on the detection of phytoplasma using nested Polymerase Chain Reaction (PCR), in potato(Solanum tuberosum L.), oil palm(Elaeis guineensis), and papaya (Carica papaya L.) plants. Phytoplasma-specific PCR amplifications were generated by nested PCR in 50% samples of Twisted neck syndrome in papaya, 38% potato samples showing Purple top symptoms, and in all the samples showing symptoms of Lethal wilt of oil palm. A continual increase in the incidence of Potato purple top was observed, and there is a high risk of contamination in the southern production zones of the country. In potato, results of this study are more closely related to Candidatus Phytoplasma subgroup 16SrI. Lethal wilt continues to be a major threat to oil palm production. In papaya, members of the group 16SrXIII-E produce a disease similar as reported in this study.
Keywords: Candidatus Phytoplasma, crop production, nested PCR
RESUMEN
Los fitoplasmas son bacterias sin pared celular limitadas al floema en plantas superiores. Las enfermedades asociadas a fitoplasmas, en el pasado, han sido poco frecuentes y severas en el Ecuador. Sin embargo, por efectos del cambio climático, su importancia se ha incrementado en un corto tiempo. Esta investigación tuvo el objetivo de detectar fitoplasmas mediante la reacción en cadena de la polimerasa (PCR) anidada, en plantas de papa(Solanum tuberosum), palma aceitera (Elaeis guineensis) y papaya(Carica papaya). Amplicones del tamaño esperado para fitoplasma fueron obtenidos en el 50% de las muestras con síndrome de cuello virado de la papaya, 38% de plantas con sintomatología de punta morada de la papa y todas las muestras con marchitez letal de la palma aceitera. Punta morada ha ido incrementándose en incidencia y severidad, y existe alto riesgo de contaminación de la enfermedad en zonas de producción de semilla al centro-sur del país. En papa los resultados de este estudio están relacionados con Candidatus Phytoplasma sp. subgrupo 16SrI. La Marchitez letal continúa siendo una seria amenaza para el cultivo de palma aceitera. En papaya, miembros del 16SrXIII-E producen sintomatología similar a la informada en el presente trabajo.
Palabras clave: Candidatus Phytoplasma, producción de plantas, PCR-anidada
INTRODUCTION
Phytoplasmas are wall-less bacteria limited to the phloem vessels in plants (McCoy et al., 1989). In Ecuador, two important diseases: potato purple top (Caicedo et al., 2015; Castillo et al., 2018) and lethal wilt of oil palm (Baer et al., 2013) are associated with phytoplasma. Besides, twisted neck syndrome of papaya could be produced by phytoplasmas but not officially reported.
Potato purple top phytoplasma has been associated with substantial economic losses in the United States and Mexico (Crosslin et al., 2006; Munyaneza et al., 2007). Various phytoplasma groups have been related to the disease: aster yellows (16SrI) subgroup 16SrI-B, peanut witches broom (16SrII), and subgroup 16SrVI-A (Lee et al., 2006). In other countries such as Bolivia, a phytoplasma belonging to the aster yellow group (16SrI) was reported by Jones et al. (2005) in potato (Solanum tuberosum L.). During 2007 and 2008, two independent surveys detected the presence of phytoplasma in potato at different geographical locations in Perú (Hodgetts et al., 2009) and reports in cultivar ‘Criolla Colombiana’ (diploid) in Colombia have implied groups 16SrV and 16SrXII (Mejía et al., 2011).
Seed transmission as well as through vectors are important factors in the epidemiology of the disease (Crosslin et al., 2006). Two species of leafhoppers (Circulifer tenelus and Macrosteles sp.) and psyllids of the genus Bactericera spp., have been reported as tentative vectors of Potato purple top in the United States and Mexico (Munyaneza et al., 2007).
Symptoms of potato purple top are chlorosis, stunting, purple discoloration of new leaves and sprouts, shortening of internodes, and formation of aerial tubers (Lee et al., 2004). Symptoms of potato purple top have been observed in potato fields of the northern provinces of Ecuador during surveys conducted in years 2012 and 2013. For instance, in Carchi and Tungurahua, the disease has been observed affecting cultivars ‘Super Chola’, ‘Gabriela’ and ‘Unica’. In less than two years, the disease has spread around the entire production zone causing economic losses close to 50% (INIAP, 2018). There is a high risk for spreading the disease around the southern production areas of the country.
Besides, in Ecuador, South, and Central America, the Lethal wilt of oil palm (Elaeis guineensis Jacq.) is an important phytopathological problem for commercial plantations. Symptoms of the disease are yellowing of new leaves, mottling ranging from orange to yellow, abortion of bunches, dieback, loss of turgor, and severe necrosis ending in the death of young and adult palms. The disease is transmitted by the hemipteran Myndus crudus Van Duzee (Howard et al., 1983; Brown et al., 2006).
In Ecuador, Lethal wilt of oil palm was first registered in 2006 (Baer et al., 2013), causing mortality in approximately 200 hectares. A surveillance made in 2013 in commercial oil palm plantations, showed 90% sampled symptomatic palms as positive for Lethal wilt (Baer et al., 2013). Phytoplasma detected in Ecuador showed 92% of homology with the strain ML (AY739023.1) reported in Colombia for Lethal wilt of oil palm by Alvarez et al. (2014). The disease has spread suddenly, and hundreds of cases are reported annually in the sanitary census carried out by commercial plantations (Asipuela-Haro et al., 2017).
Another important disease in the tropics is Twisted neck syndrome of papaya (Carica papaya L.). The disease observed in papaya plants showed symptoms such as chlorosis, reduction of apical leaves, bending of petioles and crown leaves. However, little is known about the etiology and causal agent of the disease.
There are several strains of Phytoplasma causing diseases in papaya. In Australia, Dieback, yellow crinkle and Papaya mosaic have been associated with Pphytoplasma (Guthrie et al., 1998). In the same way, the presence of Phytoplasma cells was confirmed using SEM in papaya plants of Baja California – Mexico (Poghosyan et al., 2004). Rao et al. (2011) reported members of the groups 16SrI and 16SrII affecting papaya in India. Melo et al. (2013) detected members of the subgroup 16SrXIII-E associated with apical curl necrosis in Brazil. The disease described by Melo et al. (2013) showed similarities in the symptomatology with the disease found in commercial plantations of papaya in Ecuador. In both countries, Brazil and Ecuador, the disease begins with a bending of the leaves in the bud (apical part of the extreme tip), followed by chlorosis and brown spots of leaves and peduncles.
Papaya crop is very popular and of economic importance in the Pacific coast and the Amazonian provinces of Ecuador. Traditional small-scale familiar orchards, adjacent to commercial big-monoculture plantations, make it a perfect environment for reproduction of vectors and prevalence of secondary epidemics. Additionally, papaya growers manage phytosanitary problems removing diseased plants during early symptom expression (Quito-Avila et al., 2015). Therefore, incidence and severity of several diseases, including Twisted neck syndrome, are often masked by this practice.
Molecular tools allow the detection and diagnosis of diseases in which the causal agents are obligate parasites. Nested PCR targeting the conserved 16S rDNA gene sequence allows rapid and reliable identification of phytoplasma from plant tissue samples (Lee et al., 1998). Lee et al. (1993) and Schneider et al. (1993) in two separate-independent studies showed a similar output of classification using 16SrDNA. In addition, Restriction fragment length polymorphism (RFLP) is useful for the differentiation of phytoplasma groups for an accurate identification (Rodríguez, 2007).
This research was focused on the detection of phytoplasma using nested Polymerase Chain Reaction (PCR), in potato, oil palm, and papaya plants in Ecuador. The results generates epidemiological information about phytoplasma in this country and it will be useful in complementing the understanding of Potato purple top, Papaya twisted neck syndrome and Lethal wilt of oil palm diseases.
MATERIALS AND METHODS
Sampling collection and symptoms descriptions
Two surveys were carried out in Carchi province of Ecuador (limit with Colombia) (Figure 1). Potato plants were sampled in April 2014 and June 2014. In April 2014, three potato plants with symptoms of yellowing of new leaves, purplish discoloration on the underside of the leaves, swollen nodes, broken auxiliary buds, formation of aerial tubers or abnormal production of new flushes (witches broom) were collected. Potato fields were at 2917 and 2765 meters above the sea level. Cultivars sampled were ‘Gabriela’, ‘Super Chola’ and ‘Única’. Plants were at flowering stage (150 days after planting), and applications of insecticides, Curacrón (Profenofos), Bulldock (Beta-cyfluthrin), Pirestar (Piretroid) and fungicides: Curathane 72 WP (Mancozeb + cymoxanil), Dithane (Mancozeb) and Dovex (Propamocarb), were timed each eight days, or according to the criteria of growers.
In June 2014, ten potato samples were collected in Chutan and Canchaguano locations of Carchi- Ecuador. Plants of the cultivar ‘Super Chola’ were sampled at the end of flowering and beginning of the thickening of tubers (165 days after planting). Plants of the first and the second sampling had different planting date. Negative controls of potato plants were provided by the Department of seed production of the National Agricultural Research Institute (INIAP). Negative controls were planted in the province of Pichincha-Ecuador, where symptoms of the disease not been reported (Figure 1).
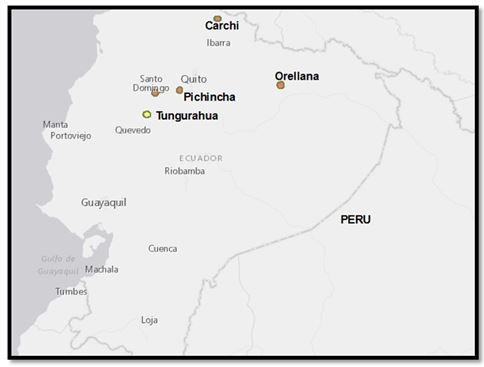
Figure 1. Map of Ecuador (not to scale) showing sampling localities (orange dots) for potato (Carchi and Pichincha), papaya (Santo Domingo), and oil palm (Orellana and Santo Domingo). Tungurahua (yellow dot) is the location where potato purple top is being spread. Source Esri, DeLorme, HERE, NGA, USGS.
In April 2012, samples of four symptomatic oil palms (in the terminal phase of the Lethal wilt disease, which is the presence of necrotic tissue in the lower leaves in adult palms) were collected in commercial plantations from Santo Domingo and Orellana provinces (Figure 1) and processed for DNA extraction (Baez et al., 2013). Extracted DNA was stored at -80 °C.
Eight samples of papaya plants were collected from commercial plots. Samples of six to seven months after transplanting were collected in the province of Santo Domingo - Ecuador, packed on ice, and transported to the laboratories of the National Department of Biotechnology, at INIAP. Samples were stored at -80°C until further processing.
DNA extraction
DNA extraction was performed using 0.1 grams of stems, aerial tubers, and leaf mid ribs plant tissue from symptomatic and asymptomatic potato plants, and 0.1 grams of mid veins of symptomatic papaya plants, and the DNA from oil palm was extracted according Baer et al. (2013). It was used as positive control. Vascular tissues from leaf veins, branches, and tubers were ground in sodium metabisulfite to avoid oxidation. DNA was extracted using protocols proposed by Ferreira and Grattapaglia (1998), Colombo et al. (1998) adapted by Piedra (Morillo and Miño, 2011), and extraction with the commercial kit PureLink Plant Total DNA (Invitrogen, Life Technologies, NY, USA), following the manufacturer instructions. After extraction, DNA was quantified in an EPOCH spectrophotometer (Biotek, Winooski, VT, USA).
DNA amplification
- Non-specific PCR with universal primers for bacteria domain
Universal primers 27-F and 1525-R for the bacteria 16S rDNA were used (Goodfellow and Stackebrandt, 1991). PCR cocktail per reaction consisted of (0.6 µl of each primer at a concentration of 50 pmol µl-1, deoxy-ribonucleotides 0.75 µl, Taq polymerase 0.15 units, MgCl2 3 µl, bovine serum albumin 0.6 µl, buffer 1X 3 µl, and molecular biology grade water 20.3 µl). Thermocycler conditions were 95 °C for 1 min, 30 cycles (94 °C for 45 s, 50 °C for 30 s, 72 °C for 30 s), final extension of 72 °C for 10 min and kept at 4 °C. The product was stored at 4 °C and agarose gels were prepared to observe bands.
- Nested PCR with specific primers for Phytoplasma
Samples of symptomatic potato plants (resembling Purple top disease), asymptomatic potato plants, symptomatic papaya plants with Twisted neck syndrome and palms plants showing Lethal wilt symptoms were amplified using nested PCR with the primers P1 - P7 in the first reaction, and R16F2n - R16R2 for the nested reaction. The last set of primers amplifies a segment of 1238 base pairs (Gundersen and Lee, 1996). In the second amplification, the product of the first was diluted 1:20. Cocktail of amplification for the first reaction was: 2.5 µl of PCR buffer (1mM), 1 µl of MgCl2 (2 mM), 0.5 µl of dNTPs (0.2 mM), 0.13 µl of BSA (0.4 ml ml-1), 1 µl of forward and reverse primers (0.4 µM) respectively, 0.1 Units Taq polymerase, 4 ng µl-1 of the product of the first PCR, and water to complete 25 µl reaction volume. For the second PCR reaction, the cocktail consisted of the same concentrations and volumes of the first one. The amplification program was variable for the temperature of annealing in the first amplification and the nested amplification (P1 - P7, 55 °C; R16F2n - R16R2, 50 °C). Amplifications were visualized in 1.5% agarose gels with ethidium bromide.
Restriction Fragment Length Polymorphism
The product of the amplification with nested PCR from symptomatic potato plants was partially digested using EcoRI and AluI. A sample from aerial tubers of potato was used for this assay. 100 µl of PCR product was obtained from agarose gels (2%) excised and cleaned using the pure link gel extraction and PCR purification kit (Invitrogen). The cocktail for digestion reaction using EcoRI (Invitrogen) was: 1 µl EcoRI, 2 µl buffer 10X, 5 µl µltra-pure water and 6.5 µl DNA (26.5 ng µl-1). The cocktail was incubated (37 °C for 1 hour, with a posterior inactivation of the enzyme (65 °C for 20 min). For AluI (Invitrogen) the reaction cocktail was: 1 µl AluI, 2 µl buffer 10X, 10.5 µl µltra-pure water and 6.5 µl DNA (26.5 ng µl-1). Conditions for incubation and inactivation of the enzyme were the same as described above. The product of both reactions was loaded in agarose (2%) and visualized in a UV trans-illuminator.
RESULTS
Plants with symptoms of Potato purple top were observed in field surveys at Carchi province. The symptomatology was similar as previously reported (Lee et al., 2006; Munyaneza et al., 2006; Crosslin et al., 2006) (Figure 2). Symptoms were observed in ‘Super Chola’, ‘Gabriela’ and ‘Unica’ cultivars. The cultivar ‘Unica’ presented symptoms; however, molecular assays were unable to amplify phytoplasma-specific PCR products of the expected size (1238 bp) in this cultivar (data not shown).
The presence of symptoms begun after flowering stage. Besides, few weeks after flowering symptoms were exacerbated and suddenly potato fields were completely cover of yellow to purple spots. The inefficiency of seed transmission appears to be the cause that certain plants do not present symptomatology even if these where planted close to symptomatic plants. The association of severe symptoms in lower altitudes, in comparison with potato fields in the higher mountain (>3500 meters above sea level), remains to be associated with a potential vector. In the ´Super Chola´ (tetraploid), a highly coveted cultivar planted in Ecuador, the disease was associated with seed tubers. However, the spreading of the disease was difficult to corroborate due to the lack of the potential vector.
Lethal wilt of oil palm disease (Baer et al., 2013) was confirmed in commercial plantations using nested PCR. Sampled oil palms were in the terminal phase of the Lethal wilt disease, which is the presence of necrotic tissue in the lower leaves in adult palms. The yellowing and orange mottling of leaves in young and mature oil palms (Figure 2), is an initial symptom of Lethal wilt.

Figure 2. Symptoms associated with diseases caused by Phytoplasma in Ecuador: (a) Twisted neck syndrome on papaya, (b) Purple discoloration of new leaves of potato, (c) Potato aerial tubers, (d) Lethal wilt initial symptoms on adult oil palm (orange discoloration of leaves).
All samples showed positive amplifications using universal 16S rDNA primers (27F and 1525R) for the bacterial domain. Nevertheless, few samples from symptomatic plants were positive for the Phytoplasma-specific amplification using nested PCR. Phytoplasma-specific PCR amplifications were generated in 50% (4 positive / 8 total) of the samples of Twisted neck syndrome in papaya, 38% of potato samples showing Purple top symptoms (5 positive / 13 total), and in all samples showing symptoms of Lethal wilt of oil palm (4 positive / 4 total) (Supplementary material Table S1).
An approximately 1238 base pair Phytoplasma-specific PCR product was obtained from symptomatic potato plants. Phytoplasma-specific PCR product was not generated from asymptomatic plants (Figure 3). The PCR product showed the same size, for phytoplasma obtained from three different hosts: Twisted neck syndrome on papaya (lane 8, Figure 3); Potato purple top (lanes 12, Figure 3) and Lethal wilt of oil palm (lane 20, Figure 3).
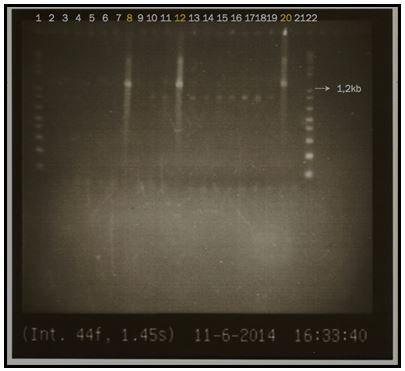
Figure 3. Nested PCR products visualized on agarose gel for: Lane 1: 1kb DNA ladder Invitrogen, lane 2: potato leaf Northern Carchi plant 2, lane 3: potato aerial tuber plant 2, lane4: potato aerial tuber plant 4, lane 5 papaya plant 2, lane6: papaya plant 5, lane 7: papaya plant 6, lane8: papaya plant 7, lane 9: potato healthy plant 1, lane10: potato leaf-plant 1, lane 11: potato aerial tuber-plant 1, lane12: potato leaf-plant 2, lane 13:potato aerial tuber-plant2, lane 14: potato leaf-plant 3, lane 15: potato aerial tuber-plant 3, lane 16 potato leaf-plant 4, lane17 potato aerial tuber plant 4, lane 18: potato leaf-plant 5, lane 19: potato aerial tuber-plant 5, lane 20: Lethal wilt of oil palm phytoplasma palm 2, lane21 water control, lane22 1kb DNA ladder.
RFLP analysis showed that the R16F2n/R2 sequence digested with AluI generated a pattern of four bands with approximate sizes of 200, 250, 330, 450 (Figure 4). A fifth band was present. However, it could represent a partial digestion or the presence of more than two copies of 16S r DNA gene.
The digestion using restriction enzyme EcoRI generated two bands with 550 and 720 base pairs (Figure 4). These bands correspond to the pattern obtained for Hosseini et al. (2011b) for Phytoplasma trifolii.

Figure 4. - Restriction patterns generated using AluI and EcoRI enzymes, with the product of the nested PCR (P1 - P7; R16F2n - R16R2) of DNA from plant tissue samples showing Potato purple top symptoms in Carchi- Ecuador.
DISCUSSION
Diseases associated with phytoplasma, in the past, have not been a serious problem in Ecuador. Nevertheless, and for climate change effects, their importance has been increasing suddenly. In this study, Phytoplasma from symptomatic potato, papaya, and oil palms were detected using nested PCR.
Dirty DNA in high concentration (>100 ng µl-1) was not a feasible alternative for nested PCR in the diagnostic of phytoplasma. In the diagnostic process it was important to consider that DNA extracted from plant tissues (midveins, aerial tubers, and stems) constitute a mixture of DNA of the pathogen and the host plant. The real concentration of the DNA of the causal agent could not be determined using conventional PCR. A common issue when using 16S amplicon studies is the amplification of chloroplasts of the host tissue. An alternative for phytoplasma detection should be the use of quantitative PCR (Ikten et al., 2016). However, certain protocols must be determined and established as routine in diagnostic clinics.
Phytoplasma-specific PCR amplifications were generated by nested PCR, showing the expected size of the band (1238 bp). The amplifications were generated in 50% samples of Twisted neck syndrome on papaya, in 38% potato samples showing Purple top symptoms, and in all samples showing symptoms of Lethal wilt of oil palm which were used in this study. These hosts (papaya, oil palm, and potato) are economically important crops in Ecuador.
In Colombia, previous reports in the diploid cultivar ‘Criolla Colombiana’, which apparently is more susceptible to phytoplasma infestation, it have shown the presence of phytoplasma using nested PCR and RFLP (Mejía et al., 2011). Phytoplasma reported in Colombia belong to groups 16SrV and 16SrXII, which are not the same reported for potato purple top in the United States (Lee et al., 2006). In potato, Candidatus Phytoplasma could be related to (16SrI) subgroup 16SrI-B, Peanut witches broom (16SrII), or 16SrVI-A as reported in United States (Lee et al., 2006). Other the groups associated with potato purple top are 16SrV and 16SrXII. Additionally, Phytoplasma trifolii (16SrVI group) has been associated with witches’ broom symptoms in potato (Hosseini et al., 2011a). Nevertheless, Phytoplasma are a large and genetically diverse group of bacteria (Lee et al., 2006).
The pattern generated differs from Lee et al. (1998) which found five bands in different sizes. Additionally, for Maize bushy stunt phytoplasma, a similar five-band pattern was generated when using AluI (Melo et al., 2013). In this sense, Josic et al. (2010) using nested PCR with universal primer pair P1-P7 followed by R16F2n - R16R2 on three cultivars of Vitis vinifera, detected similarities in the pattern reported in this study. Nevertheless, Hosseini et al. (2011b) found only three bands when they digested the product of nested PCR from Potato purple top phytoplasma. They found in a control sample for the 16SrXII group two contiguous bands at 300 to 500 bp, followed by two contiguous bands at approximately 200 bp.
In potato, restriction patterns for Phytoplasma-specific PCR products digested with AluI coincided with the group 16SrXII reported by Josic et al. (2010) and Mejía et al. (2011). Additionally, RFLP pattern observed with EcoRI, are similar to the reported by Hosseini et al. (2011b). The restriction pattern for AluI was different as the reported by the same authors. For AluI, the digestion of the product of nested PCR from symptomatic potato plants generates four fragments of 200, 250, 330 and 450 bp. This pattern was similar to the AluI pattern reported by Josic et al. (2010)for a control Phytoplasma belonging to the 16SrXII group.
Coincidences in restriction patterns digested with AluI and EcoRI, generate some questions about the group these phytoplasma belong (16SrXII as reported by Mejía et al(2011), 16SrII or 16SrVI-A, normally associated with Potato purple top in the United States (Lee et al., 2006). In this sense, Caicedo et al. (2015) found Candidatus Phytoplasma aurantifolia in association with potato in Carchi – Ecuador. The results from this study are more closely related with the subgroup presented by Castillo et al. (2018) for Candidatus Phytoplasma 16SrI.
A continual increase of Potato purple top disease was observed during the growing season 2016. Symptomatology has been observed in the province of Pichincha and Tungurahua showing that the disease is spreading, and there is a high risk of contamination in the southern production zones of the country (INIAP, 2018). Phytosanitary control agencies, research centers, as well as producers, should direct their efforts towards the quarantine and eradication of contaminated seed lots to avoid the dissemination of Potato purple top.
Due to the high rates of Potato purple top and given the proximity to seed lots, sanitary measures are being carried out to prevent the disease to spread in the seed causing a major impact in the potato industry. Although, in 2014 Pichincha province was free of the diseases in less than three years, the disease spread out over Pichincha risking some important seed producers.
Besides, Lethal wilt continues to be a major threat to oil palm production in Ecuador. A recent report showed high incidence of Lethal wilt (Phytoplasma) and sudden wilt in oil palm hybrids (Elaeis oleifera x Elaeis guineensis) in zones prone to epidemics such as the Amazon region of Ecuador (Asipuela-Haro et al., 2017).
The disease Papaya twisted neck syndrome is prevalent in all production zones of Ecuador, and the incidence of the disease has been underestimated for several years due to the elimination of trees with initial symptoms. According to observations made by field agronomists, the disease could be spread very fast if sanitary measures such as destruction of plants with initial symptoms are not implemented in commercial crops(Jorge Cueva personal communication). The recent report of Papaya apical curl necrosis (Melo et al., 2013) shows that members of the group 16SrXIII-E affects papaya and produce a disease similar as reported in this study.
CONCLUSIONS
Phytoplasma, detected using nested-PCR, are present in plants samples showing symptoms of Potato purple top, Papaya twisted neck syndrome and Lethal wilt of oil palm in Ecuador. Using nested-PCR/RFLP results, potato samples from this study showed close relatedness to Candidatus Phytoplasma 16SrI. Phytoplasma-epidemiological information compiled in this study will be useful in complementing our understanding of these three important diseases in Ecuador.
ACKNOWLEDGEMENTS
We thank to the growers that allow us to take samples in their orchards. We thank Jorge Cueva which provided technical information of the phytosanitary status of papaya crop. We thank Jairo Pastaz Zambrano for providing us samples in Canchaguano location.
Conflict of interest
None declared.
REFERENCES
Alvarez E, Mejia J, Contaldo N, Paltrinieri S, Duduk B, Bertaccini A (2014) 'Candidatus phytoplasma asteris' strains associated with oil palm lethal wilt in Colombia. Plant Dis 98(3):311-8; doi:10.1094/PDIS-12-12-1182-RE
Asipuela-Haro R, Torres A, Martínez-Rivero M (2017) Transmisión de la enfermedad marchitez sorpresiva en palma aceitera por Lincus curvatus Campos & Roelly Macropygium reticulareFabricius (Hemiptera: Pentatomidae). Revista de Protección Vegetal 32(2): 1-9
Baer N, Morillo E, Bernal G (2013) Detección del agente causal de la marchitez letal en plantaciones comerciales de palma aceitera en el Ecuador mediante técnicas de PCR y meta genómica. Disponible en: http://ciencia.espe.edu.ec/wp-content/uploads/2013/05/VID94.pdf. Consultado 26/03/2016
Brown SE, Been BO, McLaughlin W (2006) Detection and variability of the lethal yellowing group (16Sr IV) phytoplasmas in the Cedusa sp. (Hemiptera: Auchenorrhyncha: Derbidae) in Jamaica. Ann Appl Biol 149(1):53-62; doi: 10.1111/j.1744-7348.2006.00072.x
Caicedo J, Crizon M, Pozo A, Cevallos A, Simbana L, Rivera L, Arahana V (2015) First report of 'Candidatus phytoplasma aurantifolia' (16SrII) associated with potato purple top in San Gabriel-Carchi, Ecuador. New Disease Reports 32:20; doi:10.5197/j.2044-0588.2015.032.020
Castillo C, Paltrinieri S, Bustamante J, Bertaccini A (2018) Detection and molecular characterization of a 16SrI-F phytoplasma in potato showing purple top disease in Ecuador. Australasian Plant Pathology 47(3): 311-315; doi:10.1007/s13313-018-0557-9"
Colombo C, Second G, Valle T, Charrier A (1998) Genetic diversity characterization of cassava cultivars (Manihot esculenta Crantz). I) RAPD markers. Genetics and Molecular Biology 21(1): 105-113; doi:10.1590/S1415-47571998000100018
Crosslin JM, Vandemark GJ, Munyaneza JE (2006) Development of a real-time, quantitative PCR for detection of the Columbia basin potato purple top phytoplasma in plants and beet leafhoppers. Plant Dis 90(5): 663-667; doi:10.1094/PD-90-0663
Ferreira ME, Grattapaglia D (1998) Introducción al uso de marcadores moleculares en el analisis genético. Embrapa. Disponible en: http://livimagens.sct.embrapa.br/amostras/00064700.pdf. Consultado 04/05/2016
Goodfellow M, Stackebrandt E (1991) Nucleic acid techniques in bacterial systematics. Wiley, New York; doi:10.1002/jobm.3620310616
Hodgetts J, Chuquillangui C, Muller G, Arocha Y, Gamarra D, Pinillos O, Velit E, Lozada P, Boa E, Boonham N (2009) Surveys reveal the occurrence of phytoplasmas in plants at different geographical locations in Peru. Ann Appl Biol 155(1): 15-27; doi:10.1111/j.1744-7348.2009.00316.x
Hosseini P, Bahar M, Madani G, Zirak L (2011a) Molecular characterization of a phytoplasma associated with potato witches- broom disease in Iran. J Phytopathol 159(2): 120-123; doi:10.1111/j.1439-0434.2010.01732.x
Hosseini P, Bahar M, Madani G, Zirak L (2011b) Molecular characterization of phytoplasmas associated with potato purple top disease in Iran. J Phytopathol 159(4): 241-6; doi:10.1111/j.1439-0434.2010.01757.x
Howard FW, Norris RC, Thomas DL (1983) Evidence of transmission of palm lethal yellowing agent by a planthopper, Myndus crudus (Homoptera, Cixiidae). Trop Agric 60(3): 168-171
Ikten C, Ustun R, Catal M, Yol E, Uzun B (2016) Multiplex real-time qPCR assay for simultaneous and sensitive detection of phytoplasmas in sesame plants and insect vectors. Plos One 11(5): e0155891; doi:10.1371/journal.pone.0155891
INIAP (2018) INIAP ejecuta un plan emergente frente a la presencia de Punta Morada de la Papa en Ecuador. Disponible en: http://www.iniap.gob.ec/pruebav3/iniap-ejecuta-un-plan-emergente-frente-a-la-presencia-de-punta-morada-de-la-papa-en-ecuador/. Consultado 28/07/2018
Jones P, Arocha Y, Antesana O, Montellano E, Franco P (2005) ‘Brotes grandes’ (big bud) of potato: A new disease associated with a 16SrI‐ B subgroup phytoplasma in Bolivia. Plant Pathol 54(2): 234; doi:10.1111/j.1365-3059.2005.01137.x
Josic D, Kuzmanović S, Stojanović S, Aleksić G, Pavlović S, Starović M (2010) Detection of XIIA phytoplasma group on cultivar Župljanka in Župa vineyard region by RFLP analysis of 16s rDNA sequences. Genetika 42(1): 145-153; doi:10.2298/gensr 1001145J
Lee IM, Hammond RW, Davis RE, Gundersen DE (1993) Universal amplification and analysis of pathogen 16s RDNA for classification and identification of mycoplasma-like organisms. Phytopathology 83(8): 834-842; doi:10.1094/Phyto-83-834
Lee I, Bottner KD, Munyaneza JE, Secor GA, Gudmestad NC (2004) Clover proliferation group (16SrVI) subgroup A (16SrVI-A) phytoplasma is a probable causal agent of potato purple top disease in Washington and Oregon. Plant Dis 88(4): 429; doi:10.1094/PDIS.2004.88.4.429B
Lee I, Bottner KD, Secor G, Rivera-Varas V (2006) 'Candidatus Phytoplasma americanum a phytoplasma associated with a potato purple top wilt disease complex. Int J Syst Evol Microbiol 56: 1593-1597; doi:10.1099/ijs.0.64251-0
Lee I, Gundersen-Rindal DE, Davis RE, Bartoszyk IM (1998) Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. Int J Syst Evol Microbiol 48(4): 1153-1169; doi:10.1099/00207713-48-4-1153
McCoy RE, Caudwell A, Chang CJ, Chen TA, Chiykowski LN, Cousin MT, Dale JL, de Leeuw G, Golino DA, Hackett KJ (1989) Plant diseases associated with mycoplasma-like organisms. En: Whitcomb RF, Tulley JG (eds). The mycoplasmas volume V, spiroplasmas, acheloplasmas and mycoplasmas of plants and arthropods, pp. 545–640. Academic Press, San Diego; ISBN: 9780323143554
Mejía JF, Contaldo N, Paltrinieri S, Pardo JM, Ros CA, Alvarez E, Bertaccini A (2011) Molecular detection and identification of group 16SrV and 16SrXII phytoplasmas associated with potatoes in Colombia. Bulletin of Insectology 64(Supplement): 97-98
Melo L, Silva E, Flores D, Ventura J, Costa H, Bedendo I (2013) A phytoplasma representative of a new subgroup, 16SrXIII-E, associated with papaya apical curl necrosis. Eur J Plant Pathol 137(3): 445-450; doi:10.1007/s10658-013-0267-7
Morillo E, Miño GM (2011) Marcadores Molecµlares en Biotecnología Agrícola: Manual de Técnicas y Procedimientos en INIAP. Disponible en: http://repositorio.iniap.gob.ec/handle/41000/848. Consultado 21/03/2017
Munyaneza JE, Crosslin JM, Upton JE (2007) Association of Bactericera cockerelli (homoptera: Psyllidae) with "zebra chip," a new potato disease in southwestern United States and Mexico. J Econ Entomol 100(3): 656-663
Munyaneza JE, Crosslin JM, Upton JE (2006) Beet leafhopper (hemiptera: Cicadellidae) transmits the columbia basin potato purple top phytoplasma to potatoes, beets, and weeds. J Econ Entomol 99(2): 268-272; doi:10.1603/0022-0493-99.2.268
Quito-Avila DF, Alvarez RA, Ibarra MA, Martin RR (2015) Detection and partial genome sequence of a new umbra-like virus of papaya discovered in Ecuador. Eur J Plant Pathol 143(1): 199-204; doi:10.1007/s10658-015-0675-y
Rao GP, Chaturvedi Y, Priya M, Mall S (2011) Association of a 16Sr II group phytoplasma with dieback disease of papaya in India. Bulletin of Insectology 64: S106
Schneider BU, Ahrens B, Kirkpatrick C, Seemiiller E (1993) Classification of plant-pathogenic mycoplasma-like organisms using restriction-site analysis of PCR-amplified 16s rDNA. J Gen Microbiol 139: 519-527
Suplementary material
Table S1. DNA isolated from different plant tissues, under different methods of extraction, and results of the PCR amplification using universal primers (16S-rDNA), and the nested PCR (P1 - P7 / R16F2n R16R2). (bvg02119TableS1.pdf)
Recibido: 03-09-2018
Aceptado: 13-11-2018
Copyright (c) 2019 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
