Artículo original
Biotecnología Vegetal Vol. 20 No. 2: 75 - 82 , abril - junio, 2020
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Lavandula angustifolia L. plants regeneration from in vitro leaf explants-derived callus as conservation strategy
Regeneración de plantas de Lavandula angustifolia L. a partir de callos formados de explantes de hojas in vitro como estrategia de conservación
Leelavathi Devasigamani1 https://orcid.org/0000-0003-1777-8065
Raajasubramanian Devarajan2,3 https://orcid.org/0000-0001-6295-7770
Ramu oganathan1 https://orcid.org/0000-0003-2336-2031
Haseena Rafath2,4 https://orcid.org/0000-0002-0753-3454
Midhila Padman5 https://orcid.org/0000-0003-2153-5701
Govinda Raju MV6 https://orcid.org/0000-0001-5393-6556
Lavanya Giridhar2 https://orcid.org/0000-0003-4057-8651
Chetan HC7 https://orcid.org/0000-0002-1853-3353
Narendra Kuppan2* https://orcid.org/0000-0001-5637-4106
1MES Degree College of Arts, Commerce and Science. Bengaluru. Karnataka. India.
2Department of Botany, Annamalai University. Chidambaram. Tamil Nadu. India.
3Department of Botany, Thiru A Govindasamy Government Arts College. Tindivanam. Tamil Nadu. India.
4Goverment Arts College, C Muttur. Chidambaram. Tamil Nadu. India.
5Deptof Biotechnology, Sahrdaya College of Engineering and Technology. Kodakara. Thrissur. Kerala. India.
6Sanjeevini, Karnataka State Rural Lively hood Promotion Society. Sheshadripuram. Bangalore. Karnataka. India.
7Centre for Conservation of Natural Resources, The University of Trans-Disciplinary Health Sciences and Technology. Yelahanka. Bengaluru. Karnataka. India.
*Corresponding author email:narendrakuppan@gmail.com
ABSTRACT
Lavandula angustifolia L. is an aromatic and medicinal herb with multiple industrial applications. Nevertheless, the over exploitation of wild plantation attempts against to its conservation as natural resource. The aim of this paper was to regenerate plants of L. angustifolia from in vitro leaf explants by indirect organogenesis as conservation strategy. Leaves sections from in vitro plants were cultured in MS with 6-Bencylamino purine (BAP) combined with Naphthaleneacetic acid (NAA) or 2,4-Diclophenoxic acetic acid (2,4-D) to induce callus formation. The callus growth was categorized into three types according to the explant area covered by callus and the fresh and dry weigh (mg) per callus were determined. Then, callus were subcultured on MS with BAP and Kinetin combined with NAA or 2,4-D for shoots regeneration. Shoots were cultured in MS with BAP 8.88 µM, indole butyric acid (IBA) 4.92 µM and 2.68µM NAA for rooting. Plantlets were acclimatized and after 50 days of hardening the plants were transferred to the soil. Explants in all treatments formed calli. The higher percentage of callus formation with abundant growth was achieved in MS with 8.88 µM BAP + 5.36 µM NAA (92%). In MS with 4.44 µM BAP + 4.64 µM Kn+ 2.68 µM NAA, 93% of calli regenerating shoots and25-30 multiple shoots were obtained after 63 days of subculture. Shoots developed roots and plants were successfully acclimatized with 93 percentage of surviving. In vitro leaf of L. angustifolia is a suitable explant for plants regeneration.
Keywords: auxin, cytokinin, lavender
RESUMEN
Lavandula angustifolia L. es una hierba aromática y medicinal con múltiples aplicaciones industriales. Sin embargo, la sobreexplotación de las plantaciones silvestres atenta contra su conservación como recurso natural. El objetivo de este trabajo fue regenerar plantas de L. angustifolia a partir de explantes foliares in vitro mediante organogénesis indirecta como estrategia de conservación. Se cultivaron secciones de hojas de plantas in vitro en MS con 6-bencilamino purina (BAP) combinada con ácido naftalen acético (ANA) o ácido 2,4-diclorofenoxiacético (2,4-D) para inducir la formación de callos. El crecimiento de los callos se clasificó en tres tipos de acuerdo con el área de explante cubierta por el callo y se determinó la masa fresca y seca (mg) por callo. Luego, se subcultivaron los callos en MS con BAP y Kinetina (Kn) combinados con ANA o 2,4-D para la regeneración de los brotes. Los brotes se cultivaron en MS con 8.88 µM de BAP, 4.92 µM de ácido indol butírico (AIB) y 2.68 µM de ANA para su enraizamiento. Las plántulas se aclimatizaron y después de 50 días de endurecimiento se transfirieron a suelo. Los explantes en todos los tratamientos formaron callos. El mayor porcentaje de formación de callos con crecimiento abundante se logró en MS con 8.88 µM de BAP + 5.36 µM de ANA (92%). En MS con 4.44 µM de BAP + 4.64 µM de Kn + 2.68 µM de ANA, el 93% de los callos regeneraron brotes y se obtuvieron de 25-30 brotes múltiples después de 63 días de subcultivo. Los brotes desarrollaron raíces y las plantas se aclimatizaron con éxito con un alto porcentaje de supervivencia 93% La hoja in vitro de L. angustifolia es un explante adecuado para la regeneración de plantas.
Palabras clave: auxina, citoquinina, lavanda
INTRODUCTION
In nature, environment plays a pivotal role on plant species development. It may causes changes in genetic diversity, losses of many native wild species and their economically important phytochemicals. Thus, loss in genetic diversity, inbreeding and un-erupted risk is resulted in the reduction in population size of a species. In this context, the conservation of wild species is very important for genetic diversity because their extinction rate is increasing directly and indirectly in response to anthropogenic activities and by environmental factors (Frankham et al., 2002). Unavailability of agricultural land in global scenario of increase in human population and urbanization is a threat for plant diversity and agriculture. In the case of aromatic and medicinal plants, the augmented demand of biomass and metabolites attempt against the plants populations growing in natural conditions or cultivated. The use of bio-technological techniques can be a suitable alternative for conservation of these plants and supply the necessity of it use as genetically pure lines in large-scale production (Parkash and Singh, 2013).
Lavandula angustifolia L. is a widely distributed aromatic and medicinal herb worldwide (Cavanagh and Wilkinson, 2002; Hajhashemi et al., 2003). It is commonly known as Lavender or English Lavender. It belongs to the family Lamiaceae and cultivated in the Mediterranean areas. Its essential oil displays carminative, antiflatulence, and anticolic properties. Aroma therapists use it the holistic approach (Hajhashemi et al., 2003). This essential oil acts as a central nervous system depressant, anticonvulsant, sedative, spasmolytic agent, local anesthetic, antioxidant, antibacterial, and mast cell degranulation inhibitor (Cavanagh and Wilkinson, 2002). Lavender is comprised of over 100 constituents, among which the primary components are linalool and linalyl acetate, α-pinene, limonene, 1,8-cineole, cis-andtrans-ocimene, 3-octanone, camphor, caryophyllene, terpinen-4- ol, and flavonoids (Hajhashemi et al., 2003).
Vegetative propagation of Lavandula species by conventional methods is limited by low multiplication rate, poor rooting ability of the stem cuttings, as well as the lack of selected clones (Gonçalves and Romano, 2012). In this regards, the use of in vitro culture under controlled environmental conditions have been reported as alternative for overcome lavender propagation problems. Nevertheless, to produce large quantities of plants with high genetic and phytosanitary qualities, effective protocols for the in vitro propagation are required.
Different types of explants such as hypocotyl sections, apical shoots, nodal explants, leaf segments, seedlings from in vitro germinated seeds, cotyledonary buds and meristem have been used in Lavandula spp. in vitro culture (Segura and Calvo, 1991; Nobre, 1996; Dronne et al., 1999; Tsuro et al., 2000; Zuzarte et al., 2010; Miclea and Chifor, 2018; Rana et al., 2018). Among that, nodal explants are the most common explant type (Gonçalves and Romano, 2012).
Several authors referred that leaf segments of lavender plants are suitable explant for callus formation in L. vera (Tsuro et al., 2000), Lavandula × intermedia Emeric ex Loiseleur (Dronne et al., 1999) and L. angustifolia (Falk et al., 2009; Guadarrama-Flores et al., 2012). Despite of, the in vitro microbial contamination of explants taken from plants grown in field or in greenhouse is one of the problems that limited the successful of plants regeneration by organogenesis (De Bona et al., 2011).The use of explants from in vitro plants would results in regenerated plants without visible microbial contamination. Additionally, would facilitate the employ of the protocol development previously for rapid clonal propagation of L. angustifolia (Leelavathi and Kuppan, 2013), and to decrease the losses by this concept. The aim of this paper was to regenerate plants of L. angustifolia from in vitro leaf explants by indirect organogenesis as conservation strategy.
MATERIAL AND METHODS
Plant material
L. angustifolia plants were chosen and collected from the Department of Horticulture G.K.V.K., Bengaluru, India. The plants were maintained in the greenhouse at Plant Biotechnology Unit, Department of Botany, Bangalore University.
L. angustifolia was in vitro established from axillary and apical buds (Leelavathi, 2010). The apical section of leaves from in vitro plants were used as explants.
Callus formation
Nine leaves sections were inoculated in test tubes (25mm X 150 mm) with the abaxial surface in contact with the medium (Dronne et al., 1999). Culture tubes were plugged with non-absorbent cotton wool wrapped cheesecloth. The tubes containing 15 ml of basal media Murashige and Skoog (Murashige and Skoog, 1962) (MSBM) (Sigma) supplemented with 6-Bencylaminopurine (BAP) (8.88 µM) combined with Naphthaleneacetic acid (NAA) (2.68, 5.36, 8.05, 10.74, 13.42 µM) or 2,4-Diclophenoxic acetic acid (2,4-D) (2.26, 4.52, 6.78, 9.04, 11.30 µM) to induce callus formation. The pH of the media was adjusted to 5.8 before sterilization in autoclave at 121 °C and 0.1 MPa for 20 min.
Each explant was used as an experimental unit for observations and data collection and 10 replicates were utilized per treatment. After 18 days of culture, the number of explants with callus was quantified and the percentage of response was calculated.
After that, the callus were subcultured on vessels (117 mm X 271 mm, 510 ml capacity with polypropylene caps) with 40 ml of the same medium for 18 days more (36 days of culture). The growth of observed calli were categorized into three types and described as abundant (+++), moderate (++) or scanty (+) according to the explant area covered by callus. Besides, the fresh and dry weigh (mg) per callus in each treatment were determined. For dry weigh the callus were dried at 27 °C during 48 hours.
Plant regeneration
Callus were subcultured on MSBM supplemented with BAP (2.22, 4.44, 6.66, 8.88, 11.11 µM) and Kinetin (2.32, 4.64, 6.96, 9.28, 11.60 µM) combined with 2.68 µM NAA or 2.26 µM 2,4-D for shoots regeneration. The number of callus that regenerated shoots were quantified and the percentage was calculated. Besides, the total number of shoots per vessels was recorded.
After 42 days of culture, shoots were separated and cultured five per vessel (510 ml capacity) with 40 ml of MS basal medium supplemented with 8.88 µM BAP, 4.92 µM indole butyric acid (IBA) and 2.68 µM NAA for rooting. The pH of the media was adjusted to 5.8 before sterilization by autoclave at 121 °C and 0.1 MPa for 20 min.
All the cultures in callus formation and plant regeneration stages were maintained at 25±2 °C with photon flux density of 30-50 µEm-2s-1 under photoperiodic regime of 16 h light and 8 h dark cycles.
Hardening
The well-developed plantlets were carefully taken out from the culture bottles and washed thoroughly with water to remove the traces of agar. The plantlets were then transferred to plastic pots (Hardening pot capacity 2 liters with 17 cm in diameter at the top, 12 cm diameter at base and 13 cm pot height) containing potting mixture peat: perlite: vermiculate in the ratio 1:1:1 (v/v). The potted plants were covered with polythene covers to maintain humidity. These plantlets were maintained at 25 2 °C and 90-95% Relative Humidity (RH). After 15 days, the covers were removed and the plants were gradually exposed to less humid conditions. After 50 days of hardening the final survival (%) was recorded and the plants were transferred to the soil
Statistical analysis
The data obtained from the results of all experiments carried out and analyzed statistically by one-way analysis of variance (ANOVA) to determine the variation between the treatments and Critical Difference (CD). To find out variations within the treatments, the overall hypothesis of difference between more than two groups can be tested while making only on statistical decision using ANOVA.
RESULTS AND DISCUSSION
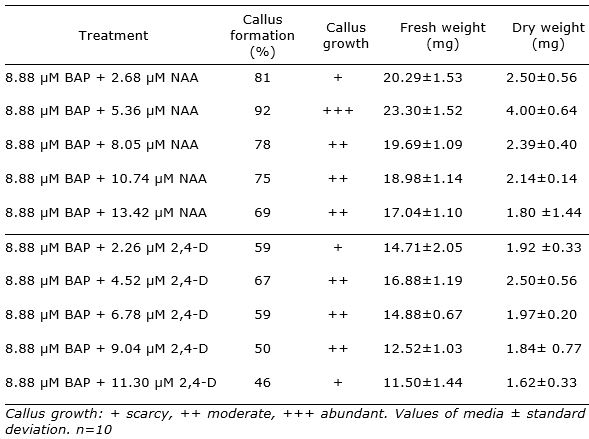
The study showed that explants in all treatments formed calli at the leaf cut ends surface after 18 days of culture. The percentage of response ranged between 46 and 92%.The best response of explants (over 75%) was recorded for the combination of BAP and NAA in MS basal medium. The higher percentage of callus formation with abundant growth was achieved in MS supplemented with 8.88 µM BAP + 5.36 µM NAA (92%) (Figure 1 b, Table 1). Despite of the in vitro leaves size is small, it morphogenetic capacity was demonstrated. In this sense, Yegorova et al. (2019) found that leaves of in vitro regenerated plants of L. angustifolia possessed differentiated mesophyll from the early stages of in vitro culture and it were characterized by a dark-green color, a lancet-like shape, and the level of necrotic tissue in them did not exceed 5–7%.
The use of in vitro leaf explants avoided the explants disinfection and reduced the incidence of microbial contaminants. In this study, the contamination percent was minimal (under 2%) and it was related to manipulation of the explants in the lab, not associated to the explants.

Figure 1. In vitro propagation of Lavandula angustifolia L. (a) Leaf explants from in vitro cultured plants inoculated on MS Basal Medium, (b) Explant showing callus initiation, (c) Callus, (d) greenish brown compact callus after 36 days of culture, (e) Callus showing shoot initiation, (f) Culture showing multiple shoots at 52 days of culture, (g) shoots after 63 days of culture, (h) Shoots in rooting stages, (i) plants hardening.
After 36 days of subculture on the same medium, large amount of profuse, greenish brown compact callus was observed (Figure 1 c, d). In the present study, in vitroleaf were chosen for retrieval of plants through callus phase. The advantage of using aseptically derived tissue was essential because the callus were free from phenolic compounds in the media. The accumulation of these compounds may inhibits organogenesis in many plants, including L. angustifolia as described by De Bona et al. (2011). The results was in accordance with reports of other researchers that have used in vitro leaf explants for callus induction in aromatic or medicinal plants (Janarthanam and Seshadri, 2008; Anjusha and Gangaprasad, 2017).
The ANOVA analysis revealed a highly significant effect for the treatment factor (p<0.05). The highest amount of callus was observed on MSBM + BAP (8.88 µM) + NAA (5.36 µM) with fresh weight 23.30 g and dry weight 4.00 g. It was observed that the increase in concentration of NAA to 8.05 µM resulted in decrease of callus formation. Besides, the combination of BAP and 2,4-D did not reached better results than BAP + NAA (Table 1).
The results were in agreement with previous report of Donne et al. (1999) whom demonstrated that callogenesis in lavandin (Lavandula × intermedia Emeric ex Loiseleur) was stimulated by the simultaneous addition of BAP and auxins in leaves explants. They informed in cultivar Grosso 2 the best results on MS medium containing 9 μM BA and 4.5 μM NAA.
Table 1. Effect of BAP with NAA or 2,4-D in MS basal medium on Lavandula angustifolia leaf callus formation and growth after 18 days of culture
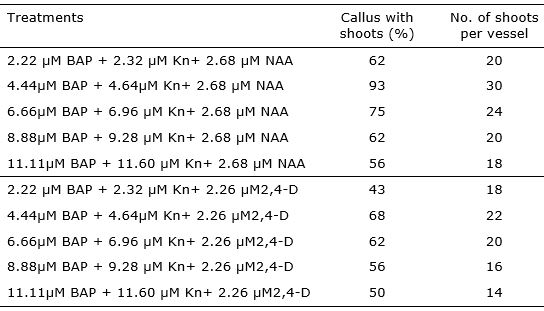
After 21 days of subculture, shoot initiation was observed on all treatments with varying frequencies of response (43 - 93%). The highest frequency of response (93%) of calli regenerating shoots was noted for those induced on the medium with 4.44 µM BAP + 4.64 µM Kn + 2.68 µM NAA (Table 2).
Shoots that formed in calli developed the green color progressively with growth after 28 days of culture (Figure 1 e and f). Two to five shoots developed only from greenish brown compact callus. Five to six multiple shoots per explant were noticed after 36 days of subculture, 10-15 after 52 days of subculture (Figure 1 f ) and 25 - 30 well developed elongated multiple shoots after 63 days of subculture (Figure 1 g).
The results of the study particularly underscore the potential of in vitro leaf explants to produce many plants in short time and space. Maximum number of multiple shoots were obtained in medium MS with 4.44 µM BAP + 4.64 µM Kn+ 2.68 µM NAA (Table 2). The average number of shoots per callus was seven in this treatment with the most efficient shoots regeneration.
It was found that composition of medium had an influence on the callus regeneration capacity. In the same way of the results in callus formation stage, the addition of 2,4-D in the media do not increase the callus response. In relation with this, Dronne et al. (1999) informed that higher level of 2,4-D in callus induction medium was unsuitable for further shoots regeneration.
Table 2. Effect of different concentrations of cytokinin and auxin for shoots regeneration in callus derived from in vitro leaf explants of Lavandula angustifolia.
Well-developed elongated multiple shoots developed roots when cultured on MS basal medium with 8.88 µM BAP + 4.92 µM IBA + 2.68 µM NAA after 30 days of culture (Figure 1 h).In contrast, De Bona et al. (2011) indicated that only in media without growth regulator or with BAP + GA3(gibberellic acid) they observed the roots induction in L. angustifolia organogenesis.
At the end of the experiment, L. angustifolia plants were successfully acclimatized (Figure 1 i) with high percentage of surviving (98%).
The propagation through in vitro leaf explants-derived callus is a reliable method for the rapid multiplication of lavender, allowing the selection, propagation and conservation of superior clones. Nevertheless, studies about the genetic stability of these plants will be develop.
On the other hand, another example of application would be the in vitro production of metabolites (Andrys et al., 2018). In this sense, Guadarrama-Flores et al. (2012) reports the presence of terpenoids linalool and linalyl acetate in callus from immature leaf explants of L. angustifolia. The extraction of valuable metabolites in lavender plants propagated by tissue culture will contribute to avoid the exploitation of wild populations (Gonçalves and Romano, 2012) and in consequence to their conservation.
There is a great need for the conservation of the plant species mainly rare and imperiled, which are economically and medicinally important. Genetic diversity is one of the key for the survival of the plant species in their natural domain. It disappearance may lead to decrease in its ability to cope with changing environment and demographic fluctuations both in short- and long-term. Once plant species disappear from their natural habitat, they cannot be regenerated and it is very difficult to re-establish their previous rich diversity. Therefore, these biotechnological tools should be applied for the conservation (Parkash and Singh, 2013) and genetic improvement of L. angustifolia.
CONCLUSIONS
In vitro leaf of L. angustifolia plants is a suitable explant for plants regeneration by indirect organogenesis as conservation strategy. It has advantage such as a reduction in microbial contamination and less phenolic oxidation.
ACKNOWLEDGEMENT
The first author is thankful to the UGC (University Grants Commission), Government of India for providing salary during the tenure of the research. UGC (University Grants Commission) had no participation in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors have no conflict of interest to declare. The work is genuinely under taken and all the data in the paper is never published or in consideration for publication in any other journal.
Author Contributions
Conceptualization MP, Data curation GR, Formal analysis RL, Funding acquisition LD, Investigation LD, Methodology CHC, Project administration RD, HR, Resources LD, Supervision MP, Validation RD, HR, MP, Visualization NK, LG, Writing - original draft NK, Writing - review & editing NK.
REFERENCES
Andrys D, Adaszyńska-Skwirzyńska M, Kulpa D (2018) Essential oil obtained from micropropagated lavender, its effect on HSF cells and application in cosmetic emulsion as a natural protective substance. Nat Prod Res 32(7): 849-853
Anjusha S, Gangaprasad A (2017) Callus culture and in vitro production of anthraquinone in Gynochth odesumbellata (L.) Razafim. & B. Bremer (Rubiaceae). Industrial Crops and Products 95: 608-614; doi:10.1016/j.indcrop.2016.11.021
Cavanagh HM, Wilkinson JN (2002) Biological activities of lavender essential oil.Phytother Res 16: 301-308
De Bona CM, Reinhart V, Biasi LA, Zanette F (2011) Lavandula dentata and Lavandula angustifolia in vitro organogenesis. Plant Cell Cult Micropropag 7(2): 66-70
Dronne S, Jullien F, Caissard JC, Faure O (1999) A Simple and efficient method for in vitro shoot regeneration from leaves of Lavandin (Lavandula x intermedia Emeric ex Loiseleur). Plant Cell Report 18: 429-433
Falk L, Biswas K, Boeckelmann A, Lane A, Mahmoud SS (2009) An efficient method for the micropropagation of lavenders: regeneration of a unique mutant. J Essent Oil Res21: 225-228
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to Conservation Genetics. Cambridge University Press, London
Gonçalves S, Romano A (2012) In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnol Adv 31: 166-174; doi:10.1016/j.biotechadv.2012.09.006
Guadarrama-Flores B, Buendía-González L, Orozco-VillaFuerte J, Estrada-Zúñiga ME, Cruz-Sosa F (2012) Producción en cultivos in vitro de los componentes principales del aceite esencial de Lavandula angustifolia. Rev Latinoamer Quím 40(2): 65-73
Hajhashemi V, Ghannadi A, Sharif B (2003) Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol 89: 67-71
Janarthanam B, Seshadri S (2008) Plantlet regeneration from leaf derived callus of Vanilla planifolia Andr. In vitro Cell Dev Biol Plant 44: 84–89; doi:10.1007/s11627-008-9123-4
Leelavathi D (2010) Ex–situ conservation of Lavandula angustifolia using in vitro techniques. Wetlands, Biodiversity and change, Bangalore
Leelavathi D, Kuppan N (2013) Protocol for rapid clonal multiplication using in vitro apical bud of Lavandula angustifolia IOSR Journal Of Pharmacy And Biological Sciences 7(3): 96-98
Miclea I, Chifor R (2018) Germination, in vitro propagation and acclimatization in Lavandula angustifolia. Bull UASVM Anim Sci Biotechnol 75: 106-109; doi:10.15835/buasvmcn-asb:2018.0017
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473-97
Nobre J (1996) In vitro cloning and micropropagation of Lavandula stoechas from field-grown plants. Plant Cell Tissue Organ Cult 46: 151-155
Parkash V, Singh H (2013) Lavandula angustifolia L. (Lavender): an important aromatic medicinal shrub and its in vitro micro-propagation for conservation. Journal of Agricultural Technology 9(3): 91-702
Rana N, Khadka S, Rajbahak S (2018) In vitro propagation of Lavender (Lavandula angustifolia Mill.). J Pl Res 16(1): 112-118
Segura J, Calvo MC (1991) Lavandula spp. (Lavender): in vitro culture, regeneration of plants, and the formation of essential oils and pigments. In: Bajaj YPS (Eds.) Biotechnology in Agriculture and Forestry, pp. 283-310, Springer Cham, Dordrech
Tsuro M, Koda M, Inoue M (2000) Efficient plant regeneration from multiple shoots formed in the leaf-derived callus of Lavandula vera, using the open culture system. SciHort 86: 81-88
Yegorova NA, Mitrofanova IV, Brailkoa VA, Grebennikovaa OA, Paliya AE, Stavtseva IV (2019) Morphogenetic, Physiological, and Biochemical Features of Lavandula angustifolia at Long-Term micropropagation in vitro . Russian Journal of Plant Physiology 66(2): 326-334
Zuzarte MR, Dinis AM, Cavaleiro C, Salgueiro LR, Canhoto JM (2010) Trichomes, essential oils and in vitro propagation of Lavandula pedunculata (Lamiaceae). Ind Crop Prod 32: 580-587
Recibido: 02-10-2019
Aceptado: 03-02-2020
Copyright (c) 2020 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
