Artículo original
Biotecnología Vegetal Vol. 21, No. 3: 177 - 188, julio - septiembre, 2020
Instituto de Biotecnología de las Plantas. UCLV. MES.
eISSN 2074-8647, RNPS: 2154
Effect of an indigenous AM and PGPR combination on chilli growth and productivity in lateritic soil
Efecto de una combinación indígena de AM y PGPR sobre el crecimiento y la productividad de chiles en suelos lateríticos
Debashis Kuila1,2 https://orcid.org/0000-0001-9353-1289
Bijoy Mal2 https://orcid.org/0000-0001-6282-1996
Sudip Mondal2 https://orcid.org/0000-0003-1717-6355
Somdatta Ghosh3* https:// orcid.org/0000-0002-2558-8520
Gunjan Biswas2 https://orcid.org/0000-0001-8776-7762
1 Mycorrhiza & Microbiology Research Section, UG & PG Department of Botany, Midnapore College (Autonomous). WB. India.
2 Department of Botany and Forestry, Vidyasagar University. Midnapore 721102. WB. India.
3 PG & UG Department of Botany, Midnapore College (Autonomous). Midnapore. WB. India.
*Corresponding Author e-mail: somdattaghosh@yahoo.co.in
ABSTRACT
Infertile lateritic soil is particularly deficient in phosphorus (P) and Nitrogen (N). Arbuscular Mycorrhiza (AM) has a key role to uptake bound P from the soil and provide to the plants growing under P-poor conditions and improve water and nutrient uptake. Azotobacter fixes free nitrogen and phosphate solubilizing bacteria (PSB) release bound phosphate, are the important groups of plant growth-promoting rhizobacteria (PGPR), sometimes they may act as mycorrhiza helper and applied with AM as biofertilizer. This pot experiment was conducted to determine the primary impact of singly and combined application of native bio-inoculants, the AM, Acaulospora, and the PGPR, Azotobacter and Pseudomonas sp. (PSB) on growth and yield of chilli (Capsicum frutescens L.), growing in acid lateritic soil. Inoculated treatments were compared for growth and productivity of chilli in terms of height, leaf number, leaf area, root collar diameter, number of flowers and number of fruits, final fresh and dry yield. The productivity of chilli showed a maximum in combined treatment of Acaulospora, Azotobacter, and PSB. Also the AM spore count and root colonization found maximum in that treatment. Hence the application of indigenous AM inoculation along with native PGPR, Azotobacter and PSB may present better productivity in low fertile lateritic soil.
Keywords: Acaulospora, Azotobacter,infertile soil, mycorrhiza, PSB
RESUMEN
El suelo laterítico infértil es particularmente deficiente en fósforo (P) y nitrógeno (N). Las micorrizas arbusculares (AM) tiene un papel clave para absorber el P unido del suelo y proporcionar a las plantas que crecen en condiciones de P pobre y mejorar la absorción de agua y nutrientes. Azotobacter que fija el nitrógeno libre y las bacterias solubilizadoras de fosfato (PSB), son grupos importantes de rizobacterias que promueven el crecimiento de las plantas (PGPR). A veces pueden actuar de conjunto con micorrizas y aplicarse con AM como biofertilizante. Este experimento en maceta se realizó para determinar el impacto primario de la aplicación individual y combinada de bio-inoculantes nativos, AM Acaulospora y PGPR Azotobacter y Pseudomonas sp. (PSB) sobre el crecimiento y el rendimiento del chile (Capsicum frutescens L.), que crece en suelo ácido laterítico. Los tratamientos inoculados se compararon para el crecimiento y la productividad del chile en términos de altura, número de hojas, área foliar, diámetro de raíz, número de flores, número de frutos, rendimiento final fresco y seco. La productividad de los chiles mostró un máximo en el tratamiento combinado de Acaulospora, Azotobacter y PSB. También el recuento de esporas de AM y la colonización de raíces encontraron el máximo en ese tratamiento. De ahí la aplicación de la inoculación de AM indígena junto con PGPR nativo, Azotobacter y PSB pueden presentar una mejor productividad en suelos lateríticos de baja fertilidad.
Palabras clave:Acaulospora, Azotobacter, suelo infértil, micorriza, PSB
INTRODUCTION
The fertility level of dry acid lateritic soil is very low. The soil is naturally deficient in phosphorus, nitrogen, and other essential nutrients like calcium, magnesium, etc. In this type of soil, nutrients remain unavailable to the plant, resulting in unfavourable conditions for plant growth (Koley, 2000). In conventional agricultural practices, uses of overdose agrochemicals day by day increasing of low fertility and toxicity in the soil in agricultural fields and pesticide-resistant pests throughout India. As a result of these real problems, adverse effects on health are being reflected (Mittal et al., 2013; Rahman and Debnath, 2015). Therefore, in modern agriculture strategy, the huge demand for producing organically toxic-free vegetables is increasing, which also cost-effective and environment-friendly (Madhusudhan, 2016; Pandey and Singh, 2012).
Mycorrhiza is the mutualistic root–fungus relationship between non-pathogenic soil fungi and the plant roots (Frank, 1885; van der Heijden et al., 2015). Arbuscular mycorrhiza (AM), belonging to the phylum Glomeromycota (Redecker et al., 2000; Kapoor et al., 2008), are the most common symbiotic association of mycorrhiza found in 90% of land plants (Schüer et al., 2001; Brundrett, 2002; Smith and Read, 2008). AM colonizes in the cortical tissue of roots during active plant growth (Kirk et al., 2001) and procures nutrient form beyond the nutrients depletion zone of roots (Li et al., 1991). Organic acids and phosphatases produced by AM help to release of available phosphorous from unavailable complexes (Bagyaraj, 1984; Entry et al., 2002; Fomina et al., 2005). They also increase nitrogen and carbon content in soil (Almas et al. , 2004). The fine extra-radical hyphae of arbuscular mycorrhizal fungi (AMF) involve in the nutrient process and uptake phosphorous (Bowen, 1973; Bucher, 2007), provide nitrogen (Madder et al., 2000; Chalot et al., 2006; Nuccio et al., 2013) by absorbing ammonium, nitrate and amino acids (Hodge et al., 2001), and deliver other plant micronutrients like copper, zinc, etc. (Smith and Read, 1997), and in exchange, withdraw organic sugar from plant roots (Kottke, 2002).
AM hyphae can absorb water from lower water potential than the plant roots (Bethlenfalvay et al., 1988; Püschel et al., 2020) and form a mycelial mat which promotes retaining soil moisture. AM symbiosis is effective to overcome low pH in acidic soil which restrict plant growth (Clark, 1997; Muthukumar et al., 2014). The increasing water and nutrient uptake ability of AMF in low fertile and dry condition (Smith and Read, 1997; Aúge, 2001) provide improved plant growth in less-nutrient soil, such as acid lateritic soil (Weber et al., 1992; Brundrett, 2009). They also protect plants from pathogenic fungi and nematode disease (Bagyaraj, 1984). Nowadays, arbuscular mycorrhizal inoculation to the crops has become an appreciable alternative that increases the growth and yield (Mishra and Verma, 1982; Johansen et al., 1993; Ghosh and Verma, 2006; Samanta and Verma, 2006; Sengupta et al., 2006; Robinson et al., 2016; Chukwuka et al., 2017).
Acaulospora is the AM fungal genus belonging to the family Acaulosporaceae, has been distributed in more than 30 species (Simanungkalit, 2006). Acaulospora sp. are a common dominant group of arbuscular mycorrhiza at the lateritic ecosystem (Ghosh and Verma, 2011). A special feature, having the sporiferous saccule with the saccule subtending hypha present in Acaulospora (INVAM, 2018).
The plant-growth-promoting rhizobacteria (PGPR), present in plant mycorrhizosphere, promote the growth of the plant (Andrade, 2004; Giri et al., 2005). Azotobacter and phosphate solubilizing bacteria are the important plant growth promoters (PGP) and act as mycorrhiza helper, having an important role in the growth of hyphae from germinating AM spores, colonization of plant roots by AM fungi and growth of external AM hyphae and dehydrogenase activity of the AM fungus (Burla et al., 1996). Azotobacter is free-living soil bacteria, fix free nitrogen directly from the environment. Inoculation of Azotobacter sp. to the plant has showed improved beneficial effects in seed germination, shoot, root biomass, and yield (Kumar et al., 2000; Kumar et al., 2009; Malik et al., 2009; Reddy et al., 2003). Phosphate solubilizing bacteria (PSB) can release bound phosphate to the available P form (Bhattacharyya and Jain, 2000; Ponmurugan and Gopi, 2006). Many research works related to plant growth promotion by the application of phosphate solubilizers have been reported earlier (Khiari and Parent, 2002; Saxena and Sharma, 2003; Yazdani et al., 2009).
Chilli(Capsicum frutescens L.) is the part of the Solanaceae family, is a very essential ingredient in our daily vegetables. It is rich in volatile oils, fatty oils, capsaicinoids, carotenoids, vitamins, proteins, fibers, and minerals and acts as a natural bactericidal, having medicinal uses to treat muscle pain, cough, asthma, sore throat, etc. (Bosland and Votava, 2000). As it is an economically important crop, the average yield of chilli in India is lower as compared to other developed countries (APEDA, 2019; FAOSTAT, 2017). Cropping in unused barren land containing infertile soil such as lateritic soil may become a suitable solution.
The present research was conducted to determine the primary impact of singly and combined application of native bio-inoculants, the AM, Acaulospora, and the PGPR, Azotobacter and Pseudomonas sp. (PSB) on growth and yield of chilli (Capsicum frutescens L.), growing in acid lateritic soil.
MATERIALS AND METHODS
Plant material
Certified seed of chilli (Capsicum frutescens L.) were collected from District Agricultural Head office, Paschim Medinipur, West Bengal, India. Healthy seeds were selected manually and surface disinfected by immersion in an aqueous solution of 0.1% (w/v) mercuric chloride (HgCl2) for 3-4 min followed by washing three times with autoclaved deionized water under aseptic conditions in a laminar flow chamber.
Experimental conditions
The experiment was conducted with chilli (C. frutescens) planted in sterilized lateritic soil (Sylvia, 1994). It was done in 22.30° N Latitude and 87.20° E Longitude in Midnapore subdivision of West Bengal in the pre-winter season. The primary soil characteristics were tested according to Jackson (1973), having pH 5.61, electrical conductivity (EC) of 0.18 m mohs/cm2, moisture content of 2.7%, organic carbon (OC) of 0.63 g kg-1, total nitrogen (N) of 0.04% and phosphate (P) of 0.03%.
Isolation of the AM propagules
Separation and isolation of indigenous AM propagules from the native ecosystem was done by following the wet sieving and decanting technique (Gerdermann and Nicolson, 1963). Spores of single species were separated morphologically using a LABOMED CSM2 microscope, and surface disinfected by 200 μg ml-1 streptomycin and 2% Chloramine-T solution (w/v)(Mosse, 1973).
The pure culture of each inoculum was cultured in sterilized sand and soil mixture. The soil: sand (1:1, v/v) mixture was sterilized by oven drying at 85 °C for 8 h with a gap of 48 h and again dried for 8 h (Sylvia, 1994). Pure cultures were done in funnels and mass cultures in surface-disinfected earthen pots, grown in an automated growth chamber with sorghum (Sorghum vulgare L.) plants. The mass cultures were maintained up to 90 days in the growth chamber at 26±2 °C under a photoperiod of 16 h light and 8 h dark, an irradiance provided by 110 W fluorescent lamps (Philips, India).
The successful mass culture of the indigenous AM inocula was identified morphologically by following Schenck and Perez (1990) and the INVAM web photo guide (INVAM, 2018) and confirmed as Acaulospora sp.
Bacterial cultures
Azotobacter was isolated from lateritic rhizospheric soil in Ashbey mannitol agar media for free-living nitrogen-fixing bacteria (Pelczar et al., 1957; Subba, 1977). Pikovskaya media for native phosphate solubilizing bacteria (Pikovskaya, 1948; Sundara and Sinha, 1963; Subba, 1977) were used. Azotobacter sp. and Pseudomonas sp. (PSB) were confirmed according to Bergey manual (Holt and Krieg, 1984; Krieg et al., 1994).
Seedling inoculation
Surface disinfected seeds of chilli were germinated in aseptic conditions and seeded with a 10 cm gap from each other in a seedling tray within a growing chamber under the environment at temperature 25-30 °C, humidity 65-70% and 15-16 h of lighting. Sterilized soil and sand mixture in 2:1 (v/v) ratio was used as growing substrate.
After germination, the healthy chilli seedlings were transferred to polythene bags (18 X 24 cm) filled with 3 kg sterilized soil (one seedling per pot) to grow for 90 days in the greenhouse. AM inoculation was done by soil-based inoculum obtained from mass culture (20 spores /g, 25 g /pot), applied under 4 cm from the soil surface. 10 ml of the bacterial cultures contained about 108 CFU ml-1 liquid medium were applied per pot.
The experiment was designed in a randomized block with eight treatments and three replicates. The treatments of this experiment consisted with T0 = No inoculation (Control), T1 = Azotobacter (AZO), T2 = Phosphate solubilizing bacteria (PSB), T3 = Acaulospora sp. (Ac), T4 = Azotobacter + PSB (AZO+PSB), T5 = Acaulospora sp. + Azotobacter (Ac+AZO), T6 = Acaulospora sp. + PSB (Ac+PSB), T7 = Acaulospora sp. + Azotobacter + PSB (Ac+AZO+PSB).
Plant parameters were measured at 30, 60 and 90 days after plantation (dap) in three replicates per treatment in terms of shoot height (cm), leaf number per plant, leaf area (cm2), the total number of flower and fruit appeared per plant, and total fresh weight (g) and dry mass (g) of fruit yield per plant. The fresh yield was calculated by summing the total fresh weight of fruits obtained. The dry mass was measured by drying the fruits in a hot air oven at 65 °C for 48 hours until a constant weight was obtained.
Rhizospheric soil samples were tested for the AM spore population by wet sieving and decanting technique (Gerdermann and Nicolson, 1963). Plant root samples were treated with 10% KOH and stained with tryphan blue (Phillips and Hayman, 1970) to study the colonization percentage by the equation 1:
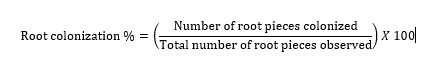
Statistical analysis
Statistical analysis of data was done by comparison of means using the least significant difference (LSD) at p < 0.05 probability, after the preforming analysis of variance (ANOVA) using IBM SPSS 20 and MS Excel 2013. Pearson correlation coefficient between variables was analyzed at p < 0.05 and p < 0.01 level of significance.
RESULTS AND DISCUSSION
The inoculation of chilli seedlings with an indigenous AM and PGPR combination increased the growth since the early stage, with prominent effect of AM.
At 30 dap, the treatment T7 having Acaulospora sp. + Azotobacter + PSB (Ac+AZO+PSB) showed height, leaf number, leaf area and root collar diameter slightly superior. Nevertheless, after 60 days of culture, the plants in treatment Ac+AZO+PSB (T7) were 23.66% higher than the control, followed by Ac+AZO (21.3%), Ac+PSB (15.5%) and Ac (10.6%). The same pattern was observed in the total leaf number and root collar diameter. Leaf area was in Ac+AZO+PSB 14.3%, in Ac+AZO 11.1% and in Ac 10.0% higher than the control.
The first appearance of flowering have few days of difference among the treatments. It was observed on 26th day after plantation in AZO+PSB and Ac+AZO+PSB, at 28th day in Ac+AZO and at 29th day in AZO, Ac, and Ac+PSB. On the other hands, first fruiting was observed at 33rd dap in AZO+PSB followed by Ac+AZO+PSB and Ac+AZO at the 36th day; and Ac and Ac+PSB at 41st day.
After 90 dap, in treatment with the combination of AM and PGPR (Ac+AZO+PSB, T7) all the parameters reached the maximum, without significantly differences with Ac+AZO (T5) in plant height, leaf number and root collar diameter. As well as T7 treatment was similar to T6 (Ac+PBS) in root collar diameter (Table 1). The shoot height in Ac+AZO+PSB was 31.64% superior to control and the leaf number 37.8%. The total fruit number was found maximum in Ac+AZO+PSB, 127.5% better than control and significantly higher (p<0.05) all the rest. A similar result was observed in the total fresh and dry mass of fruits yield per plant (Figure 1).
Table 1. Effect AM and PGPR on chilli (Capsicum frutescens L.) plants parameters after 90 days after plantation.
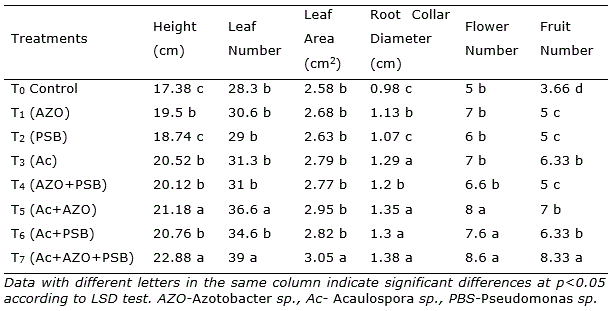
In single inoculation, the effect of AM was superior than Azotobacter and PSB in root collar diameter and fruit number. In dual inoculation, the combination of AM and Azotobacter was better than AM and PSB in height and leaf number. Plants inoculated with Azotobacter sp. have shown an increase in shoot and yield effects. It have been tested on different crops (Reddy et al., 2003; Singh and Rana, 2005; Kumar et al., 2009; Malik et al., 2009). The dual effect of inoculation of AMF and Azotobacter was found encouraging in earlier research work also (Paul et al., 2011; Dal et al., 2018; Shaimaa and Massoud, 2017). Azotobacter adds only nitrogen in soil and PSB release available phosphate, AM mainly translocate nutrients including micronutrients through the extensive mycelial network (Kayama and Yamanaka, 2014; Rouphael et al., 2015) and also release phosphates by phosphatase (Zhang et al.,2014).
Hence, AM alone is found almost sufficient for nutrition rather than Azotobacter or PSB in single inoculation. In dual treatments, also uptake ability of AM was in advance than the only supply of nutrients, and Azotobacter as a source of N and AM as P substitute worked best. Interaction between the AM, Acaulospora with Azotobacter and PSB, in this triple application may provide the development of plant growth and yield (Nadeem et al., 2014; Hashem et al., 2016). Enhanced supply of N and P in the rhizosphere by PGPR boosts up AM uptake in the triple inoculation resulting establishment of better mycorrhizal activity (Selvakumar et al., 2012; Vafadar et al., 2014; Raklami et al., 2019).
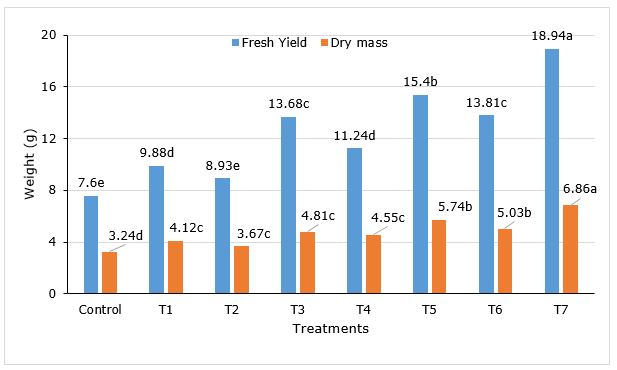
Figure 1. Fresh yield and dry mass of Capsicum frutescens L. inoculated with AM and PGPR. Treatments T1 (AZO), T2 (PSB), T3 (Ac), T4 (AZO+PSB), T5 (Ac+AZO), T6 (Ac+PSB), T7(Ac+AZO+PSB). AZO-Azotobacter sp., Ac- Acaulospora sp., PBS-Pseudomonas sp.
The mycorrhizal infection and spore population were found maximum in Ac+AZO+PSB (T7) treatment followed by MA combined with PGPR (Ac+AZO, Ac+PSB) (Figure 2). Mycorrhizal colonization was mostly mycelial and arbuscular.
Mycorrhizal colonization with class – III intensity produced in Ac+AZO+PSB and Ac+AZO. In these treatments, colonization percentage were observed higher than the inoculation formed in Ac alone. A strong significant positive correlation (r=0.997, p<0.05 and p<0.01) was observed between AM root colonization and spore density. They were also showed a significant positive correlation (p<0.05 and p<0.01) with all the measured plant parameters, including fresh yield and dry yield. The last one were also positively and significantly correlated (p<0.05 and p<0.01) with plant height.
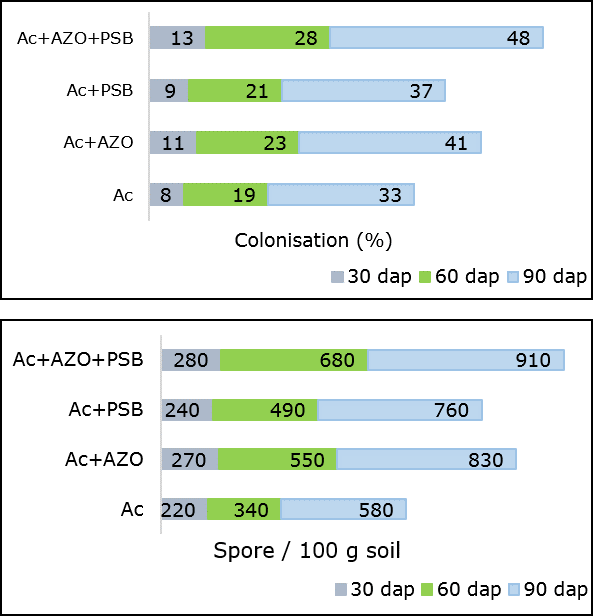
Figure 2. Mycorrhizal colonization and spore population in root and rhizosphre of chilli plant inoculates with AZO-Azotobacter sp., Ac- Acaulospora sp., PBS-Pseudomonas sp.
In all the AM related treatments, arbuscular colonization and spore formation occurred. Formation of arbuscles and high-intensity colonization in the plant roots indicates active symbiosis between Acaulospora and C. frutescens in those treatments. As the natural propagules in lateritic soil are low, the mycorrhizal application to the plant increased the growth and yield (Ghosh and Verma, 2006; Hempel et al., 2013). AM are more active in nutrient-poor dry soils, plants depended more on mycorrhizae for nutrients and moisture in this condition (Brundrett, 2009; Begum et al., 2019). The mycorrhization facilitates an effective hyphal network that spreads throughout the rhizosphere and improves the nutrient absorbing ability of the host plant (Avio et al., 2006; Püschel et al., 2020). Active mycorrhizal colonization in the plants alters some physiological processes and increases water and nutrient uptake which enhances crop growth and yield (Oyetunji et al., 2003; Mardukhi et al., 2011; Douds et al., 2005), which also influenced the chilli plant (Gaur et al., 1998; Vyas and Vyas, 2014).
The test plant, C. frutescens establish showed AM dependency on the dominant indigenous AM genus, Acaulospora, in this lateritic zone (Sieverding, 1991; Ghosh et al., 2008), which shows active adaptation and efficacy in native soil (Oliveira et al., 2005; Samanta and Verma, 2006; Akib et al., 2018). The triple application of AM, Azotobacter, and PSB were found to influence the growth of the crops too (Shwetha and Lakshman, 2013; Lallawmkima et al., 2018), though with other AM species, not indigenous or in lateritic soil.
Enhancement in productivity of chilli through the mycorrhization established in earlier works (Selvakumar and Thamizhiniyan, 2011; Bhuvaneswari et al., 2014). The mycorrhizal inoculation can enhance the yield of chilies significantly and reduced the application of chemical fertilizers by up to 50 percent (Bagyaraj and Sreeramulu, 1982). Application of AM with such mycorrhiza helping rhizobacteria develops a synergistic microbial interaction (Artursson et al., 2006) and maybe a substitute for chemical fertilizer particularly in this dry acidic soil.
CONCLUSIONS
Application of indigenous AM, Acaulospora sp. along with beneficial and native plant growth-promoting rhizobacteria, Azotobacter and PSB facilitate C. frutescens L. better growth and enhanced the yield grown in infertile dry and acid lateritic soil. Such AM and microbial consortium could be benefit other crops also in this soil type and organic agriculture and horticultural practice particularly in other poor nutrient soil and in surely reduced cost in comparison to chemical fertilizers.
ACKNOWLEDGMENT
The first author is indebted to the University Grant Commission (UGC), Government of India for the financial assistance to conduct the research work. The authors are also thankful to Prof. N. K. Verma for his comments and advice on the study.
Conflict of interest
This work was done all together and no potential conflict of interest present among the authors.
Author contributions
Conceptualization SG, Data curation DK and BM, Formal analysis DK, Funding acquisition SG, Investigation SG, Methodology DK, BM and SM, Project administration SG, Resources SG and GB, Supervision SG, Software DK and SM, Validation SG, Visualization GB, Writing—original draft DK, Writing—review & editing SG.
REFERENCES
Akib MA, Mustari K, Kuswinanti T, Syaiful SA, (2018) Identification and abundance of indigenous endomycorrhiza isolated from nickel post-mining plantation in Sorowako. Int J Curr Res Biosci Plant Biol 5(4): 8-16; doi:10.20546/ijcrbp.2018.504.002
Almas AR, Bakken LR, Mulder J (2004) Changes in tolerance of soil microbial communities in Zn and Cd contaminated soils. Soil Biol Biochem 36(5): 805-813
Andrade G (2004) Role of functional groups of microorganisms on the rhizosphere microcosm dynamics. In: Varma A, Abbott L, Werne D, Hampp R (eds). Plant Surface Microbiology, pp. 51-68. NY Springer, New York
APEDA (2019) Agri Exchange, Agricultural & Processed Food Products Export Development Authority, Ministry of Commerce and industry, government of India. Available in:http://apeda.gov.in/ Accessed 11/05/2019
Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environmental Microbiology 8: 1-10
Aúge RM (2001) Water relations, drought and vesicular – arbuscular mycorrhizal symbiosis. Mycorrhiza 11: 3-42
Avio L, Pellegrino E, Bonari E, Giovannetti M (2006) Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelial networks. New Phytologist 172: 347-357; doi:10.1111/j.1469-8137.2006.01839.x
Bagyaraj DJ, Sreeramulu KR (1982) Preinoculation with VA mycorrhiza improves growth and yield of chilli transplanted in the field and saves phosphatic fertilizer. Plant Soil 69: 375-381
Bagyaraj DJ (1984) Biological interactions with VA mycorrhizal fungi. In: Powell CL, Bagyaraj DJ (eds). VA Mycorrhiza, pp. 131-154. CRC Press, Florida
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L (2019) Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance.Front Plant Sci 10: 1068; doi:10.3389/fpls.2019.01068
Bethlenfalvay GJ, Brown MS, Ames RN, Thomas RS (1988) Effects of drought on host and endophyte development in mycorrhizal Soyabeans in relation to water use and phosphate uptake. Plant Physiol 72: 565-571
Bhattacharyya P, Jain RK (2000) Phosphorus solubilizing biofertilizers in the whirlpool of rock phosphate-challenges and opportunities. Fertilizer News 45: 45-52
Bhuvaneswari G, Reetha S, Sivaranjani R, Ramakrishnan K (2014) Effect of AM fungi and Trichoderma species as stimulations of growth and morphological character of chilli (Capsicum annuum L.). International Journal of Current Microbiology and Applied Science 3(3): 447-455
Bosland PW, Votava EJ (2000) Peppers: Vegetable and spice capsicums. CABI Publishing, Oxon
Bowen GD (1973) Mineral nutrition in mycorrhizas. In: Marks GC, Kozlowski TT (eds). Ectomycorrhizae, pp. 151-201. Academic Press, New York
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320: 1-41
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154: 275-304
Bucher M (2007) Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol 173: 11-26
Burla M, Goverde M, Schwinn FJ, Wiemken A (1996) Influence of biocontrol organisms on root pathogenic fungi and on the plant symbiotic microorganisms Rhizobium phaseoli and Glomus mosseae. J Plant Dis Prot 103: 156-163
Chalot M, Blaudez D, Brun A (2006) Ammonia: a candidate for nitrogen transfer at the mycorrhizal interface. Tren Plant Sci 11: 263-266
Chukwuka KS, Okechukwu RU, Umukoro BO (2017) Arbuscular mycorrhiza fungi, NPK (15-15-15) and cow dung interaction in sustainable cassava production and food security. Adv Plants Agric Res 7(4): 328-335; doi:10.15406/apar.2017.07.00262
Clark RB (1997) Arbuscular mycorrhizal adaptation, spore germination root colonization and lost. Plant Soil 192(1): 15-22
Dal C, Barion G, Ferrari M, Visioli G, Dramis L, Panozzo A, Vamerali T (2018) Effects of field inoculation with VAM and bacteria consortia on root growth and nutrients uptake in common wheat. Sustainability 10: 3286; doi:10.3390/su10093286
Douds DD, Nagahashi G, Pfeffer PE, Kayser WM, Reider C (2005) On-farm production and utilization of arbuscular mycorrhizal fungus inoculum. Can J Plant Sci 85: 15-21
Entry JA, Rygiewiez PT, Watrud LS, Donelly PK (2002) Influence of adverse soil condition on the formation and functioning of arbuscular mycorrhizas. Adv Environ Res 7: 123-138
FAOSTAT (2017) Chili production in 2016, Crops-World Regions-Production Quantity-Green Chillies and Peppers from pick lists, UN Food and Agriculture Organization, Statistics Division.
Fomina MA, Alexander IJ, Colpaert JV, Gadd GM (2005) Solubilization of toxic metal minerals and metal tolerance of mycorrhizal fungi. Soil Biology and Biochemistry 37: 297-299
Frank AB (1885) Ueher die aug warzel symbiose beruhende, Ernahrung gewisser Baume durch Unterirdische Plize Ber Dtsch. Bot Ges 3: 128-145
Gaur A, Adholeya A, Mukerji KG (1998) A comparison of AM fungi inoculants using Capsicum and Polianthes in marginal soil amended with organic matter. Mycorrhiza 7(6): 307-312
Gerdermann JW, Nicolson TH (1963) Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society 46: 235-244
Ghosh S, Verma NK (2006) Growth and mycorrhizal dependency of Acacia mangium Willd. inoculated with three vesicular arbuscular mycorrhizal fungi in lateritic soil. New Forests 31: 75-81
Ghosh S, Verma NK (2011) Impact of rhizospheric conditions on AM diversity, succession, and colonization in two plantations of Acacia auriculiformis and Eucalitptus tereticornis. Mycorrhiza News 22(4): 5-7
Ghosh S, Knap UK, Verma NK (2008) Effect of four arbuscular mycorrhizae on Acacia mangium Wild. Seedlings in lateritic soil. Indian J Plant Physiol 13(4): 375-380
Giri B, Giang PH, Kumari R, Prasad R, Varma A (2005) Microorganisms in soils: roles in genesis and functions. In: Buscotand F, Varma A (eds). Soil Biology, pp. 19-43. NY Springer-Verlag, New York
Hashem A, Abd Allah EF, Alqarawi AA, Al-Huqail AA, Wirth S, Egamberdieva D (2016) The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardi under salt stress. Front Microbiol 7: 1089; doi:10.3389/fmicb.2016.01089
Hempel S, Hötzenberger L, Kühn I, Michalski SG, Rillig MC, Zobel M, Moora M (2013) Mycorrhizas in the Central European Flora: relationships with plant life history traits and ecology. Ecology 94: 1389-1399
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413: 297–299
Holt JG, Krieg NR (1984) Bergey’s Manual of Systematic Bacteriology. Wiliams and Wilkins, Baltimore
INVAM (2018) Classification, International Culture Collection of Vesicular Arbuscular Mycorrhizal Fungi.
Jackson ML (1973) Soil chemical analyses. Prentice Hall, New Delhi
Johansen A, Jakobsen I, Jensen ES(1993)Hyphal N transport by a vesicular–arbuscular mycorrhizal fungus associated with cucumber grown at three nitrogen levels.Plant and Soil 160: 1-9; doi:10.1007/BF00150340
Kapoor R, Sharma D, Bhatnagar AK (2008) Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci Hortic 116: 227-239
Kayama M, Yamanaka T (2014) Growth characteristics of ectomycorrhizal seedlings of Quercus glauca, Quercus salicina and Castanopsis cuspidata planted on acidic soil. Trees 28: 569-583; doi:10.1007/s00468-013-0973-y
Khiari L, Parent V (2002) Phosphorus transformations in acid light-textured soils treated with dry swine manure. Canad J Microbiol 85: 75-87
Kirk PM, Cannon PF, David JC, Stalfers JA (2001) Ainswrth and Bisby's Dictionary of the fungi, (9th ed). CAB International, Wallingford, UK
Koley AK (2000) Basic concepts of soil science. New age international publishers, India
Kottke I (2002) Mycorrhizae-rhizosphere determinants of plant communities. In: Waisel Y, Eshel A, Kafkafi U (eds). Plant Roots, the hidden half (3rd ed), pp. 919-932. Marcel Dekker, New York
Krieg NR, Holt JG, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s Manual of Determinative Bacteriology, (9th ed). Williams & Wilkins, Baltimore
Kumar V, Aggarwal NK, Singh BP (2000) Influence of P- solubilizing analogue resistant mutants of Azotobacter chroococcum on yield and quality parameters of Helianthus annus. Folia Microbiologica 45: 347-352
Kumar V, Solanki AS, Sharma S (2009) Yield and economics of Withania somnifera influenced by dual inoculation of Azotobacter chroococcum and Pseudomonas putida. Turkish Journal of Biology 33: 219-223
Lallawmkima I, Singh SK, Sharma M (2018) Application of Azotobacter, Vesicular Arbuscular Mycorrhiza and Phosphate Solubilizing Bacteria for potato cultivation in Central Plain Zone (Pb-3) of Punjab. Journal of Environmental Biology 39: 985-989; doi:10.22438/jeb/39/6/MRN-463
Li XL, George E, Marschiner H (1991) Extension of the phosphorus depletion zone in VA – mycorrhizal white clover in calcareous soil. Plant soil 136(1): 41-48
Madder P, Vierheileg H, Boller T, Streitwalf B, Engle Freg P Chritie, Wietnken A (2000) Transport of 15N from a soil compartment separated by a polytetraflouroethylene membrane to plant roots via the hyphae of arbuscular mycorrhizal fungi. New Phytologist 146: 155-161
Madhusudhan L (2016) Organic Farming-Ecofriendly Agriculture. J Ecosys Ecograph 6: 209; doi:10.4172/2157-7625.1000209
Malik BS, Paul S, Ahlawat AK, Singh AM, Shivay YS (2009) Productivity and quality of wheat spp. grown with different fertilization condition. Indian Journal of Agricultural Sciences 79: 636-40
Mardukhi B, Rejali F, Daei G, Ardakani Md R, Malakouti Md J, Miransari Md (2011) Arbuscular mycorrhizas enhance nutrient uptake in different wheat genotypes at high salinity levels under field and greenhouse conditions. Comptes Rendus Biologies 334(7): 564-571; doi:10.1016/j.crvi.2011.05.001
Mishra RR, Verma NK (1982) Effect of different mycorrhizal treatments on the growth of onion. Acta Botanica India 11: 49-52
Mittal S, Kaur G, Vishwakarma G (2013) Effects of Environmental Pesticides on the Health of Rural Communities in the Malwa Region of Punjab, India: A Review. Human and Ecological Risk Assessment An International Journal 20: 366-387; doi:10.1080/10807039.2013.788972
Mosse B (1973) Advances in the study of vesicular arbuscular mycorrhiza. Ann Rev Phytopath 11: 171-196
Muthukumar T, Priyadharsini P, Uma E, Jaison S, Pandey RR (2014) Role of arbuscular mycorrhizal fungi in alleviation of acidity stress on plant growth. In: Miransari Md (ed). Use of Microbes for the Alleviation of Soil Stresses, pp. 43-72. Springer, New York
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32: 429-448; doi:10.1016/j.biotechadv.2013.12.005
Nuccio EE, Hodge A, Pett-Ridge J, Herman DJ, Weber PK, Firestone MK (2013) An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environmental Microbiology 15: 1870-1881; doi:10.1111/1462-2920.12081
Oliveira RS, Vosatka M, Dodd JC, Castro PML (2005) Studies on the diversity of arbuscular mycorrhizal fungi and the efficacy of two native isolates in highly alkaline anthropogenic sediment. Mycorrhiza 16: 23-31
Oyetunji OJ, Ekanayeke IJ, Osonubi O (2003) The influence of arbuscular mycorrhizae fungus, mulch and fertilizer application on the yield of yams in an agroforestry system in south western Nigeria. Maurik Bull 6: 75-82
Pandey J, Singh A (2012) Opportunities and constraints in organic farming: An Indian perspective. Journal of Scientific Research Banaras Hindu University 56: 47-72
Paul S, Singh R, Tyagi M (2011) Interactive effect with AM fungi and Azotobacter inoculated seed on germination, plant growth and yield in cotton (Gossypium hirsutum). Indian Journal of Agricultural Sciences 81(11): 1041-55
Pelczar MJ, Bard RC, Burnett GW, Conn HJ, Demoss RD, Euans EE, Weiss FA, Jennison MW, Meckee AP, Riker AJ, Warren J, Weeks OB (1957) Manual of microbiological methods. Society of American Bacteriology McGraw Hill Book Company Inc, New York
Phillips JM, Hayman DS (1970) Improved procedure for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55: 158-161
Pikovskaya RI (1948) Mobilization in phosphate in soil in concentration with vital activities of some microbial species. Microbiologya 17: 362-370
Ponmurugan P, Gopi C (2006) In vitro production of growth regulators and phosphate activity by phosphate solubilizing bacteria. African J Biotechnol 5: 348-350
Püschel D, Bitterlich M, Rydlová J(2020) Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: a Gordian knot of roots and hyphae. Mycorrhiza 30: 299-313; doi:10.1007/s00572-020-00949-9
Raklami A, Bechtaoui N, Tahiri AI, Anli M, Meddich A, Oufdou K, (2019) Use of Rhizobacteria and Mycorrhizae Consortium in the Open Field as a Strategy for Improving Crop Nutrition, Productivity and Soil Fertility. Front Microbiol 10: 1106; doi:10.3389/fmicb.2019.01106
Rahman KM, Debnath SC (2015) Agrochemical use, environmental and health hazards in Bangladesh. International Research Journal of Interdisciplinary & Multidisciplinary Studies 1: 75-79
Reddy PS, Rao TVSS, Venkatramana P, Suryanarayaana N (2003) Response of mulberry varieties to VAM and Azotobacter biofertilizers inoculation. Indian J Plant Physiol 8(2): 171-174
Redecker D, Morton JB, Bruns TD (2000) Ancestral lineages of arbuscular mycorrhizal fungi (Glomales). Mol Phylogen Evol 14: 276-284
Robinson L, Feng W, Gulbis N, Hajdu K, Harrison RJ, Jeffries P, Xu X (2016) The Use of Arbuscular Mycorrhizal Fungi to Improve Strawberry Production in Coir Substrate. Front Plant Sci 7: 1237; doi:10.3389/fpls.2016.01237
Rouphael Y, Franken P, Schneider C, Schwarz D, Giovannetti M, Agnolucci M (2015) Arbuscular mycorrhizal fungi act as bio-stimulants in horticultural crops. Sci Hort 196: 91-108; doi:10.1016/j.scienta.2015.09.002
Samanta S, Verma NK (2006) Effect of VA mycorrhiza on the growth and protein content in fruits of Capsicum annuum grown in acid lateritic soil. J Mycopathol Res 44(2): 197-200
Saxena J, Sharma V (2003) Phosphate solubilizing activity of microbes and their role as biofertilizer. In: Trivedi PC (ed). Advances in Microbiology, pp. 59-73. Scientific Publ, Jodhpur
Schenck NC, Perez Y (1990) Manual for the identification of VA mycorrhizal fungi (INVAM) (3rd ed). University of Florida, Gainesville
Schüer A, Schwarziff D, Walker C (2001) A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycological Research 105: 1413-1421
Selvakumar G, Chandrasekaran M, Charlotte S, Kiyoon K, Tongmin S (2012) Spore associated bacteria (SAB) of arbuscular mycorrhizal fungi (amf) and plant growth promoting rhizobacteria (PGPR) increase nutrient uptake and plant growth under stress conditions. Korean Journal of Soil Science and Fertillizer 45(4): 582-592; doi:10.7745/KJSSF.2012.45.4.582
Selvakumar G, Thamizhiniyan P (2011) The effect of the arbuscular mycorrhizal (am) fungus Glomus intraradices on the growth and yield of chilli (Capsicum annuum L.) under salinity stress. World Applied Sciences Journal 14(8): 1209-1214
Sengupta D, Verma NK, Ghosh BC (2006) Effect of vesicular asbuscular mycorrhiza and Rhizobium on field grown ground nut in acid lateritic soil. In: Prakash A, Mehrotra VS (Eds). Mycorrhiza, pp. 215-218. Scientific Publishers (India), Jodhpur
Shaimaa AMd, Massoud ON (2017) Impact of Inoculation with Mycorrhiza and Azotobacter under Different N and P Rates on Growth, Nutrient status, Yield and Some Soil Characteristics of Washington Navel Orange Trees. Middle East J Agric Res 6(3): 617-638
Shwetha C, Lakshman HC (2013) Effect of AM fungi, Azotobacter and phosphate solubilizing bacteria in improvement of Amaranthus paniculatus L. - A leafy vegetable. Research Journal of Biotechnology 8: 36-39
Sieverding E (1991) Vesicular-arbuscular mycorrhizal management in tropical agro system. German Technical Co-operation (GZT), Eschborn
Simanungkalit RDM (2006) Arbuscular Mycorrhiza Fungi. Available in: http://www.balittanah.litbang.pertanian.go.id Accessed 14/06/2019
Singh CS, Rana JPS (2005) Arbuscular mycorrhizal fungi. In: Kaushik B D (ed). Advances in microbiology at IARI 1961-2004, pp. 123-34. Mounto publishing house, New Delhi
Smith SE, Read DJ (1997) Mycorrhizal Symbiosis. Academic Press, London
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, (3rd Edition). Academic Press, London
Subba NS (1977) Soil Microorganisms and Plant Growth. Oxford and IBH Publishing Co, New Delhi
Sundara WVB, Sinha MK (1963) Phosphate dissolving micro-organisms in the soil and rhizosphere. Indian J Agric Sci 33: 272-278
Sylvia DM (1994) Vesicular-arbuscular mycorrhizal fungi. In: Weaver RW, Angle S, Bottomley P, Bezdicek D, Smith S, Tabatabai A, Wollum A (Eds). Methods of soil Analysis, (Part 2) Microbiological and Biochemical properties, pp. 351-378. Soil Science Society of America, Madison; ISBN: 9780891188650
Vafadar F, Amooaghaie R, Otroshy Md (2014) Effects of plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. Journal of Plant Interactions 9(1): 128-136; doi:10.1080/17429145.2013.779035
van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205: 1406-1423
Vyas M, Vyas A (2014) Field Response of Capsicum annuum Dually Inoculated with AM Fungi and PGPR in Western Rajasthan. International Journal of Research Studies in Biosciences 2(3): 21-26
Weber E, George E, Beck DP, Saxena MC, Marschner H (1992) Vesicular arbuscular mycorrhizal and phosphorus uptake in chickpea grown in Nothern Syria. Expl Agric 28: 433-442
Yazdani M, Bahmanyar MA, Pirdashti H, Esmaili MA (2009) Effect of Phosphate solubilization microorganisms (PSM) and plant growth promoting rhizobacteria (PGPR) on yield and yield components of Corn (Zea mays L.). Proc World Acad Science Eng Technol 37: 90-92
Zhang L, Fan JQ, Ding XD, He XH, Zhang FS, Feng G (2014) Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol Biochem 74: 177-183; doi:10.1016/j.soilbio.2014.03.004
Recibido: 16-04-2020
Aceptado: 22-05-2020
Copyright (c) 2021 Biotecnología Vegetal
Biotecnología Vegetal eISSN 2074-8647, RNPS: 2154. ISSN 1609-1841, RNPS: 0397 Editada por: Instituto de Biotecnología de las Plantas. Universidad Central Marta Abreu de Las Villas. Carretera a Camajuaní km 5.5, Santa Clara, Villa Clara, Cuba CP 54 830 Tel: 53 42200124, e-mail: info@ibp.co.cu

Biotecnología Vegetal está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
